Social Media Used By Half Of US Plastic Surgeons To Market Their Practice

George C. Peck Jr. MD FACS
Board Certified Plastic & Reconstructive Surgeon
Weighing Hormone Replacement: Risks & Benefits
By RONI CARYN RABIN
Nearly a decade ago, researchers in Boston decided to see whether older men who were not in very good shape could benefit from daily doses of testosterone.
The scientists recruited several hundred volunteers and gave them the hormone or a placebo. Those taking testosterone got stronger, compared with those taking the placebo, and they could carry a load up stairs faster.
But they also had nearly five times the number of cardiovascular problems, including heart attacks and strokes, and safety monitors ended the trial early.
Since those findings were published in 2010, studies of testosterone treatment have produced mixed results. A 2012 study of veterans aged 40 and over with low testosterone found that those treated with the hormone were less likely to die, but more recent reports, including one published last week, have documented an increase in cardiovascular risk in men over age 65 taking testosterone, as well as in younger men with a history of heart disease.
Officials at the Food and Drug Administration said on Friday that they were reassessing the safety of testosterone products in light of the recent studies, and will investigate rates of stroke, heart attack and death in men using the drugs.
In recent years, testosterone has been heavily promoted as a cure-all for low energy, low libido, depression and other ills among middle-aged men. “Low T” is a ubiquitous diagnosis, heard in television commercials and locker rooms.
Between 2001 and 2011, hormone use by men 40 and over nearly quadrupled. By the end of that period, nearly one in 25 men in their 60s was taking testosterone.
Though the drug is indicated for men with abnormally low testosterone levels, a condition called hypogonadism, doctors have been prescribing it to many men with normal levels.
“For people with truly low testosterone levels, the benefits outweigh the risks,” said Dr. Brad Anawalt of the University of Washington in Seattle and an author of the study that found testosterone could help certain veterans. “But for millions of others, it’s in the same category as snake oil.”
Many physicians have become more willing to prescribe testosterone to people who don’t fit the classical diagnosis of hypogonadism and have only borderline low levels that may be related to normal age-related hormonal declines, he said. Testosterone levels begin to decline by about 1 percent a year in men at age 30.
“There are what I would label testosterone factories out there, and it’s terrifying because we don’t know what the long-term safety profile is,” Dr. Anawalt said.
Some critics say the trend is reminiscent of another hormonal regimen with a sexy allure — estrogen, or as it was commonly called, hormone replacement therapy — which was widely promoted to menopausal women for decades based on scanty evidence of benefit and inadequate scrutiny of the potential harm.
“We’re giving people hormones that we don’t know they need for a disease that we don’t know they have, and we don’t know if it’ll help them or harm them,” said Dr. Lisa Schwartz, a professor at the Dartmouth Institute for Health
Policy and Clinical Practice, who wrote a 2013 paper about the marketing of low testosterone as a disease in need of a treatment.
Hormone treatment for women — typically a combination of estrogen and synthetic progesterone, unless the woman has undergone hysterectomy — is used to treat menopausal symptoms such as hot flashes, mood swings and low sexual desire. But for years physicians were convinced that estrogen protected women from heart disease, and promoted it as a long-term preventive regimen.
Some 20 percent of menopausal women were taking hormones by the time the Women’s Health Initiative finally put the hypothesis to the test in a large government-sponsored clinical trial.
The results stunned experts: Rather than protecting women from heart disease, the popular estrogen and progestin combination increased the risk of blood clots, strokes and breast cancer when compared with a placebo, and did not protect from heart disease.
Now there are calls for a similar, government-funded clinical trial to establish the benefits and risks of testosterone hormone treatment for men. The National Institutes of Health is sponsoring a large randomized controlled trial, called the T trial, designed to see whether older men who take testosterone actually experience better physical, sexual and cognitive function, and whether the hormone reduces risk factors for heart disease and diabetes.
The trial is not designed to determine the long-term risks of treatment, but rather to determine whether the treatment has health benefits. Though low testosterone is associated with health problems in older men, such as bone loss, decreased strength and decreased sex drive, it is not clear that low testosterone is the cause of these problems or that boosting testosterone reverses them, said Dr. Ronald Swerdloff of the David Geffen School of Medicine at U.C.L.A.
Testosterone also declines in men who are obese and don’t exercise, Dr. Anawalt pointed out.
Mary Schooling, a Hunter College professor of public health, is convinced testosterone is harmful for older men, and a trial like the Women’s Health Initiative would be a mistake. “It’s in the pharmaceutical companies’ interest to have a trial going on for 10 years,” she said. “In that time, they can continue to sell testosterone.”
Dr. Anawalt has been taking a firm approach with his patients, often telling them that he’s not convinced that they really suffer from low testosterone. “There are so many men out there looking for the elixir of youth,” he said.
“I say, ‘I’m not going to prescribe a therapy for you for the rest of your life if I’m not sure it’s safe for you. And by the way, if you could exercise a little more, lose a couple pounds and eat more healthfully — there’s evidence you can raise your testosterone that way.’ ”
© 2014 The New York Times Company
 a Diane S. Berson, MD, FAAD,b Joel L. Cohen, MD, FAAD,c Wendy E. Roberts, MD,d Isaac Starker, MD, FACS,e and Beatrice Wang, MD, FRCPC, FAADf
a Diane S. Berson, MD, FAAD,b Joel L. Cohen, MD, FAAD,c Wendy E. Roberts, MD,d Isaac Starker, MD, FACS,e and Beatrice Wang, MD, FRCPC, FAADfChemical peeling is a popular, relatively inexpensive, and generally safe method for treatment of some skin disorders and to refresh and rejuvenate skin. This article focuses on chemical peels and their use in routine clinical practice. Chemical peels are classified by the depth of action into superficial, medium, and deep peels. The depth of the peel is correlated with clinical changes, with the greatest change achieved by deep peels. However, the depth is also associated with longer healing times and the potential for complications. A wide variety of peels are available, utilizing various topical agents and concentrations, including a recent salicylic acid derivative, β-lipohydroxy acid, which has properties that may expand the clinical use of peels. Superficial peels, penetrating only the epidermis, can be used to enhance treatment for a variety of conditions, including acne, melasma, dyschromias, photodamage, and actinic keratoses. Medium-depth peels, penetrating to the papillary dermis, may be used for dyschromia, multiple solar keratoses, superficial scars, and pigmentary disorders. Deep peels, affecting reticular dermis, may be used for severe photoaging, deep wrinkles, or scars. Peels can be combined with other in-office facial resurfacing techniques to optimize outcomes and enhance patient satisfaction and allow clinicians to tailor the treatment to individual patient needs. Successful outcomes are based on a careful patient selection as well as appropriate use of specific peeling agents. Used properly, the chemical peel has the potential to fill an important therapeutic need in the dermatologist’s and plastic surgeon’s armamentarium.
Chemical peels are used to create an injury of a specific skin depth with the goal of stimulating new skin growth and improving surface texture and appearance. The exfoliative effect of chemical peels stimulates new epidermal growth and collagen with more evenly distributed melanin. Chemical peels are classified by the depth of action into superficial, medium, and deep peels.1 Specific peeling agents should be selected based on the disorder to be treated and used with an appropriate peel depth, determined by the histological level or severity of skin pathology to maximize success. However, other considerations, such as skin characteristics, area of skin to be treated, safety issues, healing time, and patient adherence, should also be taken into account for best overall results.
Chemical peels are very common in clinical practice. The American Society of Plastic Surgery reported that more than one million peel procedures were performed by its members in 2008.2 Although peels have recently had an upsurge in research interest,3 they are best performed and/or supervised by dermatologists and plastic surgeons who have far more experience and knowledge with cosmetic procedures than other physicians.3
Using the correct depth chemical peel is a critical component for success. Superficial peels affect the epidermis and dermal-epidermal interface. They are useful in the treatment of mild dyschromias, acne, post-inflammatory pigmentation, and AKs and help in achieving skin radiance and luminosity. Because of their superficial action, superficial peels can be used in nearly all skin types. After a superficial peel, epidermal regeneration can be expected within 3 to 5 days, and desquamation is usually well accepted. Superficial peels exert their actions by decreasing corneocyte adhesion and increasing dermal collagen.1These peels are a good method for rejuvenating the epidermis and upper dermal layers of skin.
Medium-depth peels may be used in the treatment of dyschromias, such as solar lentigines, multiple keratoses, superficial scars, pigmentary disorders, and textural changes. The healing process is longer, with full epithelialization occurring in about one week. Sun protection after a medium-depth peel is recommended for several weeks. Because of the risk of prolonged hyperpigmentation, medium-depth peels should be conducted with caution in patients with dark skin.
Deep peels may be used for severe photoaging, deep or coarse wrinkles, scars, and sometimes precancerous skin lesions. Usually performed with phenol in combination with croton oil, deep peels cause rapid denaturization of surface keratin and other proteins in the dermis and outer dermis. Penetrating the reticular dermis, the deep peel maximizes the regeneration of new collagen. Epithelialization occurs in 5 to 10 days, but deep peels require significant healing time, usually two months or more, and sun protection must always be used. Phenol is rapidly absorbed into the circulation, potentiating cardiotoxicity in the form of arrhythmias. Therefore, special care, such as cardiopulmonary monitoring and intravenous hydration, must be provided to address this concern.4,5Other complications include hypopigmentation, hyperpigmentation, scarring, and keloid formation, which may occur primarily with phenol peels (similar to laser resurfacing, the occurrence of these problems is both operator- and technique-dependent).6 Phenol peels are primarily performed in operating room settings and are frequently used as adjuncts to surgical procedures. Due to the increased risk of prolonged or permanent pigmentary changes, deep peels are not recommended for most dark-skinned individuals. Currently, new laser techniques are a popular alternative for major deep skin resurfacing because they avoid the adverse effects of deep chemical peels, even if phenol is used in lower concentrations.
Chemical peels are a mainstay in the cosmetic practitioner’s armamentarium because they can be used to treat some skin disorders and can provide an aesthetic benefit. In addition, chemical peels may be readily combined with other resurfacing and rejuvenation procedures, often providing synergistic treatment and more flexibility in tailoring treatments to specific patient needs and conditions. Clinicians can customize regimens to the patient’s individual needs using several modalities, such as at-home skin regimens, chemical peels, and lasers or dermabrasion, to provide unheralded flexibility in individualized care.
This brief review covers chemical peels and their role in appropriate indications by combining evidence-based medicine with the clinical experience of the authors. The recent introduction of β-lipohydroxy acid, a salicylic acid derivative with antibacterial, anti-inflammatory, antifungal, and anticomedogenic properties, may provide additional therapeutic benefit, and thus its role is highlighted.
A wide variety of peels are available with different mechanisms of actions, which can be modulated by altering concentrations. Agents for superficial peels today include the alpha hydroxy acids (AHAs), such as glycolic acid (GA), and the beta hydroxy acids (BHAs), including salicylic acid (SA). A derivative of SA, β-lipohydroxy acid (LHA, up to 10%) is widely used in Europe and was recently introduced in the United States. Tretinoin peels are used to treat melasma and postinflammatory hyperpigmentation (PIH).7 Trichloroacetic acid (TCA) can be used for superficial (10–20%) peels and for medium-depth peels (35%). Combination peels, such as Monheit’s combination (Jessner’s solution with TCA),8 Brody’s combination (solid carbon dioxide with TCA),9 Coleman’s combination (GA 70% + TCA),10 and Jessner’s solution with GA,11 have been used for medium-depth peels where a deeper effect on the skin is required but deep peeling is not an option. Deep peels are typically performed with phenol-based solutions, including Baker-Gordon phenol peel and the more recent Hetter phenol-croton oil peel.12
The recent introduction of LHA is important because it not only provides efficient exfoliation at low concentrations, it possesses antibacterial, anti-inflammatory, antifungal, and anticomedonic properties.13–15 An SA derivative with an additional fatty chain, LHA has increased lipophilicity compared to SA, for a more targeted mechanism of action and greater keratolytic effect.13 LHA has good penetration into the sebaceous follicle and through the epidermis, but it penetrates less deeply into the skin than GA or SA (LaRoche-Posay; data on file; 2008) interacting with the more superficial layers of the stratum corneum, specifically the compactum/disjunctum interface. Thus, its activity focuses on the follicle and epidermis. LHA has a pH similar to normal skin (pH 5.5) and has proven to be quite tolerable. Conveniently, the LHA peel does not require neutralization in contrast to a GA peel.
LHA has an interesting mechanism of action. It targets the corneosome/corneocyte interface to cleanly detach individual corneosomes, which may partially explain skin smoothness after an LHA peel, since it minimizes desquamation of clumps, which leads to roughness.14 These effects are visible to the naked eye.13 Similar to SA, LHA does not affect keratin fibers or the corneocyte membrane.13 AHAs and BHAs do not modify corneocyte keratins. The clean and uniform corneocyte separation achieved with LHA more closely mimics the natural turnover of skin. SA and GA can result in only partial detachment of some cells, which leads to uneven exfoliation of cells in clumps. The differences between LHA, SA, and lactic acid with regard to epidermal effects are summarized in Table 1. The histological section of skin samples treated with LHA also shows targeting of the horny layer by LHA along with good epidermal integrity. Studies have demonstrated that LHA targets corneodesmosome protein structures, particularly corneodesmosine, in the horny layer (LaRoche-Posay; data on file; 2008). While SA has the same target, its activity is less specific and is limited to arbitrary intercellular cleaving of some intercellular junctions. Finally, AHAs have far less affinity for these proteins and the less drastic cleaving of the intercellular bonds of SA leads to less precise desquamation than that observed with LHA.
Other properties of LHA include modifying the stratum corneum so that postpeel, it is thinner, flexible, and resistant to wrinkling and cracking.16 In-vivo immunohistological study of LHA peels showed increased epidermal thickness and dendrytic hyperplasia without markers of irritation or inflammation.15 Thus, LHA has similar effects to those of SA on epidermal indices, such as thickness of stratum corneum and germinative compartment and number of nuclei.14,17 Additionally, LHA-treated older skin has been shown to recover some physiological characteristics of younger skin, such as more rapid cell cycling.14 LHA has very few side effects. In clinical studies, LHA peels were well tolerated with some patients experiencing burning and crusting after the initial peel. No cases of PIH or scarring have been reported with LHA.18
Acne. Clinicians and patients often use chemical peels as an adjunct to medical therapy in acne because they produce complementary rapid therapeutic effects and improvements in skin appearance and textures.19,20 The primary effect may be on comedones with a concomitant reduction in inflammatory lesions (Figures 1–3). Peels may allow topical acne agents to penetrate more efficiently into the skin and may improve PIH.21 With good technique, peels may also be beneficial for dark-skinned patients who have pigmentary changes due to acne.20 While 2009 American Academy of Dermatology guidelines suggest that more evidence is needed to determine best practices,22 clinical experience has shown promising utility. Peels that have been studied for active acne include SA, GA, LHA, and Jessner’s solution.



SA. SA can be used to treat comedones and inflammatory lesions.21 In the early 1980s, a controlled, double-blind trial (N=49) showed that low concentrations of SA (0.5–3%) helped speed resolution of inflammatory lesions.23 Later, Lee et al18 reported improvement in acne in 35 Korean patients with acne treated with SA 30% peels, and that the reduction in lesion counts increased as the duration of peel continued.18 SA has shown good effects in dark-skinned Asian, African-American, and Hispanic patients with acne.24,25 In addition, this treatment regimen facilitated resolution of PIH as well as a decrease in the overall pigmentation of the face.25
Most recently, Kessler et al26 compared 30% GA versus 30% SA peels in 20 patients with mild-to-moderate acne using a split-face design. Peels were performed every two weeks for a total of six treatments. Both peels improved acne; however, the authors found that the SA peel had better sustained efficacy (number of acne lesions, improvement rating by blinded evaluator) and fewer side effects than GA, presumably due to the increased lipophilicity of SA.26 Overall, the authors of this paper agree with the impression that SA peels are better tolerated than GA peels in acne patients.
LHA. Due to its lipophilicity, LHA targets the sebum-rich pilosebaceous units and has a strong comedolytic effect. Uhoda et al27 studied LHA in acne-prone women and women with comedonal acne (n=28) in a randomized, controlled, clinical trial. As shown with ultraviolet (UV) light video recordings and computerized image analysis, both the number and size of microcomedones were significantly decreased in 10 of 12 LHA-treated patients versus 3 of 10 untreated controls. In addition, image analysis showed a marked reduction in the density of follicular keratotic plugs. As microcomedones resolved, there was also a decrease in follicular bacterial load. There were no reported side effects with LHA use.27
The previously described anticomedogenic properties of LHA include loosening of both intercorneocyte binding and bacterial adhesion inside the follicular openings16 and thinning of the stratum corneum.28LHA reduced the bacterial population per volume of follicular cast by 21±13 percent following daily treatment with a 2% cream. In addition, bacterial viability was reduced.28,16
GA. GA may be used in acne to normalize keratinization and increase epidermal and dermal hyaluronic acid and collagen gene expression.29 It has been studied in concentrations ranging from 35 to 70%.19,30.31 GA 70% has been shown to reduce comedones in Asian patients.19Lower concentrations (35% or 50%) also achieved significant resolution of both inflammatory and non-inflammatory acne lesions.30 Another study also conducted on Asian patients showed improvement in pigmentation problems and reported that acne flares after the first treatment diminished with subsequent treatments.30 A case series suggested that comedones may improve more readily than inflammatory lesions,31 but this remains to be validated.
Jessner’s solution. Superficial Jessner’s solution peels have been used to manage acne. Medium-depth peels involving Jessner’s solution plus TCA have also been used to treat mild acne scarring. Kim et al19compared Jessner’s solution versus GA 70% in patients with facial acne in a split-face study (n=26). Efficacy was similar between the two types of peels, but Jessner’s solution was associated with a significantly greater degree of exfoliation compared with GA (P<0.01).19 Lee et al32 studied the effect of GA and Jessner’s solution on facial sebum secretion in patients with acne.32 GA 30% or Jessner’s solution peels were performed twice at an interval of two weeks in 38 patients (27% GA, 11% Jessner’s solution), and sebum levels were measured. In this study, neither type of peel changed sebum secretion after two peels.32 However, Jessner’s solution may be an option for superficial peeling as an adjunctive treatment in patients with acne.
Acne scarring. Acne scars are polymorphic; therefore, it is important to assess and design treatment according to the types of scars, while also keeping in mind patient expectations. Chemical peels, laser resurfacing, dermabrasion, and fractionated laser technology as well as fillers and subcision are commonly used modalities for acne scar therapy. From a peel standpoint, patients with mild-to-moderate acne scarring may be treated. Peels that have been used include SA, GA, TCA, LHA, and Jessner’s solution. Peels are used as an adjunct to medical therapy including a retinoid or AHAs.33Studies of Jessner’s solution in combination with TCA in medium-depth peels have also shown benefit in acne scarring.34,35 Medium-depth and deep-depth phenol peels, while useful for treatment of acne scarring, are not recommended for dark skin types IV to VI due to a high risk of permanent pigmentary changes.36 Regional dermabrasion is an effective adjunct to chemical peel for medium-depth scars.37
Phenol solutions. Deep chemical peels may be used to treat acne scarring. The most common solutions are combinations of phenol and croton oil.12, 38–40 These solutions penetrate to the midreticular region and maximize the production of collagen.41 Park et al42 used a modified phenol peel, which was applied to 46 patients of Asian descent, 11 of whom were treated for acne scarring and 28 for wrinkles. Seven of 11 patients (64%) with acne scars improved 51 percent or more based on physician and patient assessment. The most frequent side effect was PIH (74%).42
Photodamage. Photodamaged skin is associated with chronic UV light exposure. Photoaging changes include a thicker dermis due to breakdown of the elastic fiber network and a thinner epidermis having cellular atypia. Often, the result can be irregular pigmentation, wrinkling, loss of elasticity, development of solar lentigines and actinic keratoses, and coarseness. Histologically, peels alter the epidermis creating a more normal pattern with columnar cells showing return of polarity, more regular distribution of melanocytes, and melanin granules. A wide range of chemical peels including AHA, SA, TCA, and phenol are used to treat photodamage; selection is based on patient presentation and severity of photodamage. The efficacy of treating photoaging with tretinoin is well established.16 Efficacy of peels to treat photodamage has also been repeatedly reported. In photodamaged skin, peels cause skin exfoliation and rejuvenation,43 and repeated superficial peels may be used.44 With advanced photoaging changes, a peel may be combined with laser resurfacing or other procedures.
AKs are precancerous lesions that are also a result of chronic UV exposure. Peels have been used to treat AKs and are appropriate treatment for most regions of the body. Chemical peels can eliminate AKs and may be able to provide prophylaxis for a prolonged time period.45 They have also recently shown clinical benefit when AKs were observed in combination with Bowen’s Disease.46
SA. Kligman et al47 studied SA 30% in regimens of single and multiple peels at four-week intervals and reported improvement of pigmentation, skin texture, and reduction of fine lines in patients with moderately photodamaged skin. Humphreys et al48 reported that 40% TCA (a borderline medium-depth peel) plus topical retinoid treatment improved solar lentigines, AKs, and skin texture, but had minimal effect on wrinkles.
GA. Rendon et al49 described the use of superficial GA peels in combination with dermal fillers and botulinum toxin, successfully addressing wrinkles, uneven skin tone, skin laxity, and skin clarity. They used a schedule that separates fillers and peels by approximately one week; with botulinum toxin, the peel was administered after the toxin in the same visit or the procedures were separated by one or more days to minimize the potential for side effects.49 Briden et al50 reported good patient satisfaction when using superficial GA peels with microdermabrasion in photoaging.
LHA. Efficacy of LHA peeling in photodamage was shown in a randomized, intraindividual-controlled, split-face trial evaluating LHA (5–10%) versus GA peel (20–50%) (LaRoche-Posay; data on file; 2008). A total of 43 women with fine lines, wrinkles, and hyperpigmentation were treated with six applications with both acids over nine weeks. Both treatments showed a significant effect in reducing fine lines, wrinkles, and hyperpigmentation (Figure 4). However, the efficacy of four LHA sessions was equivalent to six sessions of GA. The LHA peel was well tolerated. No patient withdrew from the study, and the most common side effect was transient erythema that persisted for less than two hours (LaRoche-Posay; data on file; 2008).

Leveque et al14 assessed skin improvement in 80 women who were treated with an excipient containing LHA 1% daily for six months, finding a progressive improvement in complexion, with an onset of action occurring within one month. In a randomized, controlled trial comparing GA 10% versus LHA 2% versus retinoic acid 0.05% on the forearm, LHA and retinoic acid improved surface texture similarly while GA had a very minimal effect.51
AHA increases UV sensitivity,52,53 while LHA increases the skin’s resistance to UV-induced damage. Saint-Leger16 reported that the minimal erythema dose was 210mJ/cm2 versus 140mJ/cm2 for untreated and placebo-treated controls (LaRoche-Posay; data on file; 2008).16 This protective effect may be due to the antioxidant properties of LHA, which can inactivate the oxygen singlet (1O2) without reacting with it and thus quench the superoxide anion. It also reacts avidly with hydroxyl radicals to produce 2,5-dihydrobenzoic acid, an excellent scavenger of the superoxide anion (L’Oreal; data on file; 2008).16
Combination solutions. Lawrence et al54 conducted a 15-patient, split face study comparing a medium-depth chemical peel consisting of Jessner’s solution and 35% TCA with topical fluorouracil in the treatment of widespread facial AKs. Both treatments reduced the number of visible AKs by 75 percent and produced equivalent reductions in keratinocyte atypia, hyperkeratosis, parakeratosis, and inflammation, with no significant alteration of preexisting solar elastosis and telangiectasia.54 Also, a 70% glycolic peel and a 5% 5-fluorouracil solution (Drogaderma, Sao Paulo, Brazil) was used in actinic porokeratosis every two weeks for four months with benefit, but the results remain to be validated.55
Phenol solutions. A study by Chew et al56 suggested that that there was a greater improvement in upper-lip wrinkles with Baker’s phenol chemical peel than with CO2 laser treatment (p<0.03), although the change from baseline was statistically significant for both chemical peel and CO2 laser. In basal cell carcinoma, Kaminaka et al57 demonstrated that nevoid basal cell carcinoma could successfully be treated with phenol and TCA peeling.57 A more recent study by Kaminaka et al46 not only demonstrated a significant benefit of the phenol-base peel in patients with AKs and Bowen’s Disease, but also identified biomarkers that assisted in predicting clinical success from failure. They studied 46 patients treated with phenol peels and followed up for one or more years. Biopsy specimens were taken before and after treatment. In this small but important study, 39 patients (84.8%) had a complete response after 1 to 8 treatment sessions. Statistical differences also correlated the number of treatment sessions with histology, personal history of skin cancer, tumor thickness, and cyclin A expression. The authors concluded that tumor thickness and cyclin A could be specific and useful biomarkers as an accurate therapeutic diagnosis tool, thus providing a more useful way to measure potential therapeutic benefit.46
Melasma. Patients with melasma usually present with irregular patches of darkened skin on the cheeks, forehead, upper lip, nose, and chin.58 Melasma has always been very challenging to treat for multiple reasons including the presence of melanin at varying depths in the epidermis and dermis. Because chemical peels remove melanin and improve skin tone and texture, they are commonly used in treating this condition. More superficial and more limited involvement melasma is often more responsive to treatment. Data from small studies suggest that melasma improvement occurs more rapidly when peels are combined with medical therapy. Several peels have been studied (SA, LHA, GA, TCA, tretinoin and resorcinol, retinoic acid and Jessner’s), although GA is currently most popular.
SA. Grimes25 reported that a series of five SA peels at concentrations of 20 to 30% plus hydroquinone at two-week intervals resulted in moderate-to-significant improvement in 66 percent of six darker skinned (V–VI) patients. The treatment was well tolerated, and there was no residual hypo- or hyperpigmentation.25 In unpublished data, Grimes noted that SA peels without hydroquinone preparation were associated with hyperpigmentation. Because of the known propensity of darker skin to develop dyschromias, Grimes recommended that even superficial peels be used with care and caution.
GA. In a study of GA 30 to 40% peels plus a modified Kligman’s formula (retinoid, corticosteroid, and hydroquinone) versus Kligman’s formula alone (n=40), Sarkar58 found a significant decrease in Melasma Area and Severity Index (MASI) score from baseline to 21 weeks in both groups. Figure 5 shows an 80-percent change in score at Week 21 in the peel group and a 63-percent change in the control group (P<.001).58 However, the addition of a peel achieved a significantly greater effect versus the control group of Kligman’s formula alone (more rapid and greater improvement, P<.001).58 Erbil et al61 studied serial GA peels (from 35–50% and 70% every second peel) plus combination topical therapy (azelaic acid and adapalene) in 28 women with melasma59 and found better results in the group receiving chemical peels plus topical therapy (P=0.048), but only when the GA concentration was 50% or higher.59 GA peels in concentrations of 20 to 70% administered every three weeks were studied alone or in combination with a topical regimen of hydroquinone plus 10% GA in 10 Asian women60 in which the combination trended toward significance (P>0.059).
In another study, a triple combination cream consisting of fluocinolone acetonide 0.01%, hydroquinone 4%, and tretinoin 0.05% was used in an alternating sequential treatment pattern, cycling with a series of GA peels, for the treatment of moderate-to-severe melasma.61 Spectrometry measurements of the difference in melanin for involved versus uninvolved skin confirmed that hyperpigmentation was significantly reduced at Weeks 6 and 12 compared with baseline (P<0.001 for both), with evaluations showing 90-percent improvement or more by Week 12 with the treatment approach.61
TCA. Kalla et al62 compared 55 to 75% GA versus 10 to 15% TCA peels in 100 patients with recalcitrant melasma. They reported that both the time to response and degree of response were more favorable with TCA compared with GA; however, relapse was more common in the TCA group (25 vs. 5.9% in the GA group).62 Soliman et al63 reported that 20% TCA peels plus topical 5% ascorbic acid was superior to TCA peeling alone in 30 women with epidermal melasma.
Other peels. An early report by Karam64 used a 50% solution of resorcinol in patients with melasma and skin types I to IV.64 A more recent study of 30 patients with mostly Fitzpatrick type IV skin type were treated successfully with lactic acid in a split-face comparison with Jessner’s solution (N=30). All patients showed significant improvement as calculated by MASI score before and after treatment.65Khungar et al described a pilot study in which serial 1% tretinoin peels were as effective a therapy for melasma in dark-skinned individuals as 70% GA.7
Potential side effects of peels. Superficial peels are safe and tolerated with mild discomfort, such as transient burning, irritation, and erythema.66 Scarring is rare in superficial peels, as are PIH and infection. In medium and deep peels, lines of demarcation that are technique related can occur. Care should be taken to feather peel solution at junctions with nonpeeled skin to avoid this effect. Side effects of deeper peels can also include pigmentary changes (e.g., PIH for dark-skinned individuals), infections, allergic reactions, improper healing, hypersensivity, disease exacerbation, and those due to improper application.67,68
Care must also be taken to prophylactically treat patients with a history of herpes simplex infections. Herpetic episodes, usually on the lip or above the vermilion border, may be prevented with prophylactic oral acyclovir, valacyclovir hydrochloride, or famciclovir.69,70 Antiviral agents are especially useful in patients who indicate a strong history of multiple herpetic lesions each year.
The best way to prevent complications is to identify patients at risk and maintain an appropriate peel depth that balances efficacy with known adverse events. Patients at risk include those with PIH, keloid formation, heavy occupational sun exposure, a history of intolerability to sunscreens, and uncooperative patients.
Tolerability of peels may be influenced by many factors, such as peel agents, concentration, depth, skin type, and concomitant use of skin care products. PIH can be exacerbated by sun exposure, so it is important to educate patients and closely monitor their recovery phase. Sunscreens should be used continuously to limit PIH development. Epidermal PIH responds well to various treatments, while dermal PIH remains problematic. Pretreatment with bleaching agents before beginning therapy with peels decreases the appearance of PIH. Treatment options include hydroquinone or kojic acid or other tyrosinase inhibitors.
In medium and deep peels, a common location of scarring is on the lower part of the face,71 due perhaps to greater tissue movement or more aggressive treatment. Other rare causes of scarring include infections and premature peeling, making post-peel monitoring an essential component of management. Delayed healing and persistent redness are early warning signs, and treatment with topical antibiotics and potent topical corticosteroids should be initiated as soon as possible to minimize scarring. Resistant scars may be treated with dermabrasion or pulsed dye laser followed by silicone sheeting therapy.
Acneiform eruptions may occur during or after peeling, presenting as erythematous follicular papules. These eruptions respond to oral antibiotics used in acne treatment. Discontinuation of oily skin preparations is also recommended.
Milia usually appear 2 to 4 months after peels in up to 20 percent of patients undergoing medium and deep peels and may be treated with extraction or electrosurgery.
Medium-depth peels are associated with most of the complications described above, though most can be managed successfully. Medium- and deep-depth peels should be used with great caution on skin types IV to VI. Toxicity, although rare, has been reported with resorcinol, SA, and phenol deep peels.72
Indications for peeling in dark-skinned patients include treatment of dyschromia, PIH, acne, melasma, scarring, and pseudofolliculitis barbae. Clinicians should evaluate the Fitzpatrick skin type and ethnic background as part of the process of selecting whether a peel is an appropriate therapy and which peel is best suited for the individual patient.73 Different ethnicities may respond unpredictably to chemical peeling regardless of skin phenotype. An individual patient history of PIH is very important to take into account. Hexsel et al74 point out that Latin-Americans and Hispanics have a diverse range of skin phototypes and pigmentation and are prone to an increased incidence of melasma and PIH. In this subpopulation, they recommend peels as second-line therapy after topical therapies fail.74
Superficial peels may be safely used in patients with dark skin, including LHA 5 to 10%, TCA 10 to 20%, GA 20 to 70%, SA 20 to 30%, lactic acid, and Jessner’s solution. In addition, variations of peel technique may be used, including spot treatment of PIH. This may be performed with TCA 25%, Jessner’s solution, SA, and LHA. Table 2 provides recommended agents for peeling in dark-skinned individuals by specific indication. Deep phenol peels are not recommended for dark skin types IV to VI due to the high risk of prolonged or permanent pigmentary changes.75 However, Fintsi et al76 described safe use of phenol-based peels in patients with olive and dark skin and dark eyes and hair.76
Medical history. Taking a complete history prior to peeling is critical. It can enhance aesthetic results by identifying any factors that may contribute to problems and provides an opportunity to discuss adherence issues necessary for successful management.67,77 It is important to gain insight into patients’ perceptions of wound healing and scar formation, as well as prior experience with resurfacing procedures or facelift surgery.67 Current literature recommends waiting at least six months after discontinuing oral isotretinoin therapy before performing resurfacing procedures.67
A current medication list should be obtained, and photosensitizing agents should be discontinued. Some dermatological conditions, including rosacea, seborrheic or atopic dermatitis, and psoriasis, may increase the risk for postoperative problems, such as disease exacerbation, excessive and/or prolonged erythema, hypersensitivity, or delayed healing.67 Prophylactic antiviral agents should be prescribed as required.67 Since sun protection after peeling is essential, discussion in relation to the patient’s past habits and experience is important.
Pretreatment. Pretreatment can help to enhance outcomes and is often started 2 to 4 weeks prior to the peel and discontinued 3 to 5 days before the procedure.21 Topical retinoids or a prepeel solution can help to create a smooth stratum corneum to achieve a more even penetration of the peel. Topical retinoids may also speed healing.1 Humphreys et al48 reported that pretreatment with a topical retinoid resulted in more rapid and even frosting as well as a decrease in telangiectasias, which the authors postulated as being due to deeper penetration of TCA with retinoid pretreatment.48
Before a chemical peel, hydroquinone may be used to reduce the likelihood of PIH in dark-skinned individuals.1 Discussing peel after-effects with patients before the peel is also important to aid comprehension of the peeling process.
Postpeel, patients should use a broad-spectrum sunscreen on a daily basis and implement a gentle cleansing regimen with toner and peel serum as prescribed. Moisturizers may also be recommended.
Maintenance. After a chemical peel, edema, erythema, and desquamation may occur for 1 to 3 days for superficial peels and 5 to 10 days for medium to deep peels. A cleansing agent may be used and antibacterial ointment applied especially for deep peels. Patients should be instructed to avoid peeling or scratching the affected skin and to use only simple moisturizers.
A long-term maintenance program will preserve the results of chemical peels in most patients. Patient participation and education is required, emphasizing the importance of sun protection and the use of appropriate skin care regimens that include cleansing, toning, exfoliation, and moisturizers. Patients need to have realistic expectations and understand that achieving benefits from peels requires repeated procedures. If the peel regimen works well for the patient, clinicians should consider a maintenance protocol, which may be one peel per month for six months, then every three months thereafter depending on the need and the season. Topical retinoid maintenance therapy can also help maintain the skin rejuvenation results achieved with a chemical peel. It may be used alone on a daily or intermittent basis or in addition to 2 to 3 weekly light peels periodically. Maintenance regimens may also include products with combinations of kojic acid, hydroquinone, LHA, SA, GA, or ascorbic acid.
Importance of tailoring therapy. It is important to develop a peel program that is tailored to the individual needs of the patient. For example, a patient with visible photodamage who can tolerate social and work downtime may be treated with a 35% TCA peel while another patient may be better treated with a series of lighter peels to minimize downtime. In addition, patients who are treated with peels may also be interested in a variety of other treatments, such as botulinum toxin or fillers, to improve the signs of aging.
Chemical peels remain popular for the treatment of some skin disorders and for aesthetic improvement. Peels have been studied and shown to be effective as treatment for a myriad of conditions including acne, superficial scarring, photodamage, and melasma. Patients who are willing to undergo continued treatment are likely to be the best candidates. Newer molecules such as the LHA superficial peel provide unique characteristics including targeted action and should be studied further. Clinicians should remember that there can be excellent synergy between peels and other procedures. Chemical peels are most effectively used in combination with a topical, at-home regimen, which, depending on the condition, may include exfoliating or moisturizing products, bleaching agents, or retinoids. Using peels less frequently but on a continuing basis is beneficial to help keep improvement ongoing, especially for superficial peels. Medium peels and deep peels are used more judiciously over time, but can address particularly difficult conditions effectively over the course of several treatments. Finally, it is important for patients to maintain a good sun protection regimen to optimize the clinical results achieved with chemical peels.
Collagen is the most abundant protein in mammals, accounting for around 30% of the protein content of the human body. It is often considered to be the “glue that holds the body together”.
Collagen is found in fibrous tissues such as skin, ligaments and tendons, as well as in the bones, blood vessels, the cornea of the eye, and in the gut.
Collagen is vital for strengthening blood vessels and giving skin its elasticity and strength. The degradation of collagen causes wrinkles and other skin issues. As a result, collagen is one of the most popular supplements among the elderly – because of it’s skin healing properties.
This Medical News Today information article provides details on the characteristics of collagen, its functions, the link between collagen and old age, and medical advances.
Collagen has very good tensile strength – it is one of the long fibrous structural proteins that gives cells structure from the outside, as well as supporting the majority of the body’s tissues.
As an amino acid, collagen is made from the amine (-NH2) and carboxylic acid (-COOH) functional groups. The main elements of collagen are hydrogen, oxygen, nitrogen, and carbon.
Collagen contains three-stranded helical segments of similar structure. The rare abundance of the three amino acids glycine, proline, and hydroxyproline, give collagen its triple-helical structure.1
The composition of collagen is considered unique given its high hydroxyproline content.
There are over 28 different types of collagen. Collagen fibers give strength and structure to many different parts of the body. It is one of the main components of the extracellular matrix, which is the defining feature of connective tissues in humans and other mammals.2
Collagen is necessary for conserving the youthfulness of skin and attenuating wrinkles, it is also essential for the elasticity of the connective tissue of the skin, allowing it to expand and contract without damaging any tissue.3
When we get older, the production of collagen begins to slow down and cell structures start losing their strength.
As a result, skin starts to become fragile, less elastic and wrinkles set in. In addition, hair starts losing its color, joints lose their flexibility, and bone quality begins to deteriorate.
Millions of people worldwide seek out ways to stimulate the production of collagen when wrinkles start to show.
Injectable skin fillers are becoming increasingly popular for getting rid of the lines and wrinkles associated with aging.
According to a study published in Archives of Dermatology, injections with “dermal fillers” contain hyaluronic acid, which is thought to stimulate the production of collagen, restoring the structure of damaged skin.4
Treatment for heart disease – scientists found that collagen is able to transform from its rigid form into a more flexible state and then back again. Their findings have enabled scientists to develop drugs that can reduce the risk of heart attack by preventing collagen from rupturing in arterial plaques.
Gum healing – a novel method using bovine collagen has been shown to be able to enhance gum healing, according to an article published in the journal Head & Face Medicine.5
Arthritis – A strong and stable alternative to human collagen was developed by a group of researchers at the University of Wisconsin-Madison, which could be used to treat conditions caused by collagen defects such as arthritis.6
Written by Joseph Nordqvist

More and more Britons are turning to cosmetic surgery to improve their appearance, with the number of procedures topping 50,000 a year for the first time in 2013.
A total of 50,122 operations were carried out last year, 17% more than in 2012. Demand grew despite the scandal over potentially hazardous PIP breast implants and grew by levels unseen since before the recession began in 2008.
Breast enlargement remains the most popular procedure, with 11,135 augmentations performed in 2013 – up 13% year-on-year – according to figures collected by the British Association of Aesthetic Plastic Surgeons (BAAPS).
Anti-ageing treatments were the second and third most popular, with 7,808 blepharoplasty (eyelid surgery) operations carried out – a rise of 14% – and 6,380 face or neck lifts undertaken (up 13%).
In all 5,476 people (up 13%) underwent breast reduction, while 4,878 (up 17%) had a rhinoplasty to improve the shape of their nose. While the 10 most common surgeries all saw double-digit rises, the biggest jump was in fat-removing liposuction operations. A total of 4,326 people had the procedure, up 41% in a year, reflecting the relentless rise in obesity levels.
The market for cosmetic procedures remains predominantly female. Women had 45,365 operations, compared with 4,757 for men.
BAAPS president Rajiv Grover said confidence in cosmetic surgery had returned “with Britons choosing to spend on procedures with proven track records, such as liposuction”.
He added: “Whether it is breast augmentation or anti-ageing procedures like facelifting, the public are choosing tried and tested surgical methods rather than the magical-sounding quick fixes that fail to deliver results.”
However, patient dissatisfaction is growing alongside rising demand, warned Sally Taber, chair of Independent Healthcare Advisory Services. She said: “It is important to measure patient satisfaction rates as well as numbers of operations carried out.
“While it may be that the majority of procedures were wholly satisfactory, the Independent Sector Complaints Adjudication Service (ISCAS), the body responsible for adjudicating on complaints in the independent healthcare sector, has seen an increase in complaints from patients who have undergone cosmetic surgery during the same period. This needs further attention.”
The Huffington Post | By Rebecca AdamsPosted: 01/28/2013 2:43 pm EST | Updated: 01/28/2013 6:23 pm EST
When the curvy Kate Upton was asked by GQ what her one wish would be, she replied, “I would have smaller boobs. Just kidding! Hahaha.” We can only assume this sentiment is shared by our other fellow Americans. Our friends across the pond, however, have different tastes when it comes to breast size — and there are statistics to prove it.
According to audit figures from the British Association of Aesthetic Plastic Surgeons, the demand for breast augmentation, the most popular procedure, dropped by 1.6 percent in 2012. That might not sound like much. But at the same time, the plastic surgeries that rank just below the top slot are increasing in the double digits and fast.
Take eyebrow lifts, for example. The anti-aging procedure’s popularity increased by a whopping 17 percent last year — meaning 1,812 people last year turned their attention upwards. Perhaps Brits are giving the area a little more attention because of Kate Middleton‘s sought-after scouse brow? Hey, The Duchess Effect has caused similar reactions…
Other top procedures include eyelid surgery (up 13 percent), face and neck lifts (also up 13 percent) and fat transfer (also up 13 percent, but we’re not quite sure what that one entails). And ready for some good news? Liposuction went down 14 percent. Basically, British women had more fat injected into their bodies than removed last year. Who knew?
Moral of the story: Fake breasts are out and arched eyebrows are in. Sorry, Kate Upton, it looks like the Kate that Brits want to emulate is the one that’s married to Prince William. Can you blame them?
What do you think of these findings? Do you think there’s a different plastic surgery aesthetic stateside?
Click over to the British Association of Aesthetic Plastic Surgeons’ website to see the rest of their findings.
Expert Rev Dermatol. 2013;8(5):565-580.
Aging of the skin is a multifactorial phenomenon in which ongoing intrinsic changes combine the cumulative effects of chronic exposure to the elements, primarily UV radiation, in a synergistic fashion, causing the skin to lose its thickness and elasticity and develop wrinkles. There is now an increased interest in a wide range of non-ablative treatments for skin aging, which are used to rejuvenate skin with minimal downtime and complications. As the demand for minimally invasive rejuvenation is increasing, different modalities have been designed to produce favorable alterations in the dermis with no epidermal damage via photomodulation, selective photothermolysis, fractional photothermolysis, radio waves, electro-optical synergy, injectable fillers, neurotoxins, skin needling and biorejuvenation to stimulate collagen synthesis and rejuvenate the aged skin while preserving the integrity of the epidermis.
Aside from being the largest organ of the human body, skin is also the only organ continually exposed to the surrounding world, interacting with the environment and reflecting the general health condition and age changes. [1]
Understanding the mechanisms by which the skin ages has been increasing significantly, along with considerable progress on the way to prevent and reverse the visible signs of aging. However, there are still several mysterious factors concerning aging process and why we all appear to age differently. [2] Aging of the skin is likely caused by both intrinsic (biologic) ‘intrinsic aging’, and extrinsic (environmental) factors ‘extrinsic or photoaging’; these factors are interconnected and may share a final common pathway. [3] The quality of skin features is greatly affected by aging, as skin ages, it tends to become roughened, lax and wrinkled with some telangiectasia and pigmentary changes. [1,4,5]
The main histological feature of photodamaged skin is solar elastosis; with accumulation of elastotic material in the papillary and middle dermis. Meanwhile, photoaged skin shows gradual decrease in collagen content. [6] Additionally, collagen network becomes disordered with decreased synthesis and enhanced breakdown. [7] These changes contribute to the skin laxity and wrinkling formation. [8]
Besides being an art, facial rejuvenation is a developing science. Patients now routinely present to their physician requesting information on improving the signs of facial aging; it is the physician’s responsibility to select the most appropriate intervention(s) based on the patient’s age, physical needs and concerns, extent and location of volume loss and cosmetic goals. [9,10] Different therapeutic approaches were used throughout the years to give the face a youthful appearance. However, because each person is unique, there is no one modality that is best for everyone. [11] Therefore, to choose the most appropriate therapy, distinctions must be done between rhytides caused by loss of collagen within the dermis, wrinkles due to volumetric loss of fat, redundant folds created by gravitational pull and those caused by hyperfunctional facial muscles. [12]
For ease of patient education, the treatment options for addressing these changes may be simplified into five categories, often referred to as the ‘5 Rs (Redraping, Resurfacing, Retaining, Relaxing and Refilling) of skin rejuvenation’: surgically Redraping and lifting redundant tissue; Resurfacing photoaged skin with ablative or non-ablative technologies whether physical, chemical or mechanical; Retaining with skin care; Relaxing dynamic rhytides that are due to hyperfunctional muscles with neurotoxins and Refilling of diminished subcutaneous tissue by restoring 3D volume. [13,14]
Although ablative modalities remain the gold principle for photodamaged skin rejuvenation, its use is associated with significant risk of side effects as well as a prolonged and an unpleasant post-treatment ‘downtime’ and recovery period. [15]Thus, interest in ablative treatment has waned considerably while non-ablative modalities as well as fractional skin rejuvenation have become appealing alternative treatments. [16]
New perspectives in non-ablative skin rejuvenation treatments have been established with the development of new technologies and techniques, which are used to rejuvenate skin with minimal downtime and complications. [3,17] Many different terms have been used to describe these procedures including: subsurface resurfacing, laser toning and minimally invasive skin rejuvenation. These modalities are designed to produce many cosmetic benefits, including improvement of wrinkles, skin laxity and texture. [18]
Beside lasers and various in-office procedures, many topical skin care agents were used for prophylaxis as sun screens and for rejuvenation such as retinoic acid and different anti-oxidants including vitamins C and E, co-enzyme Q10 and green tea.[19]
Choosing the appropriate treatment modality which will be the key to success in skin rejuvenation depends on careful evaluation and determining the patient’s needs, skin type and condition, to frame a treatment plan. [20] Good candidates for minimally invasive techniques tend to have minimal facial sagging. Patients should understand that skin texture will improve and fine lines will be softened but not eradicated. Cumulative aesthetic benefits will occur gradually and will be less dramatic than those seen with ablative resurfacing. [18] Patients with Fitzpatrick skin type III or less are generally best candidates for different procedures with minimal risk of complications. [21,22]
The goal of most minimally invasive treatments is to induce selective dermal injury which results in wound repair response; while keeping the overlying epidermis intact. [18] In response to the induced dermal injury, the healing process begins to stimulate the fibroblast with deposition and reorientation of collagen bundles. [23] Such modalities for skin rejuvenation could be classified into two types, the first relates to treatment of ectatic vessels, pigmentation and pilosebaceous changes, while the second refers to dermal remodeling with wrinkle reduction and/or skin tightening. [24,25]
Minimally invasive skin rejuvenation techniques could be categorized into several different general modalities including: non-ablative laser technologies and light sources, non-laser modalities (radiofrequency [RF] systems and ultrasounds), electro-optical synergy (ELOS) technique beside other approaches and procedures (superficial chemical peels, microdermabrasion, injectable fillers, neurotoxins, skin needling, mesotherapy, platelet-rich plasma [PRP] and stem cell therapy) (Figure 1).
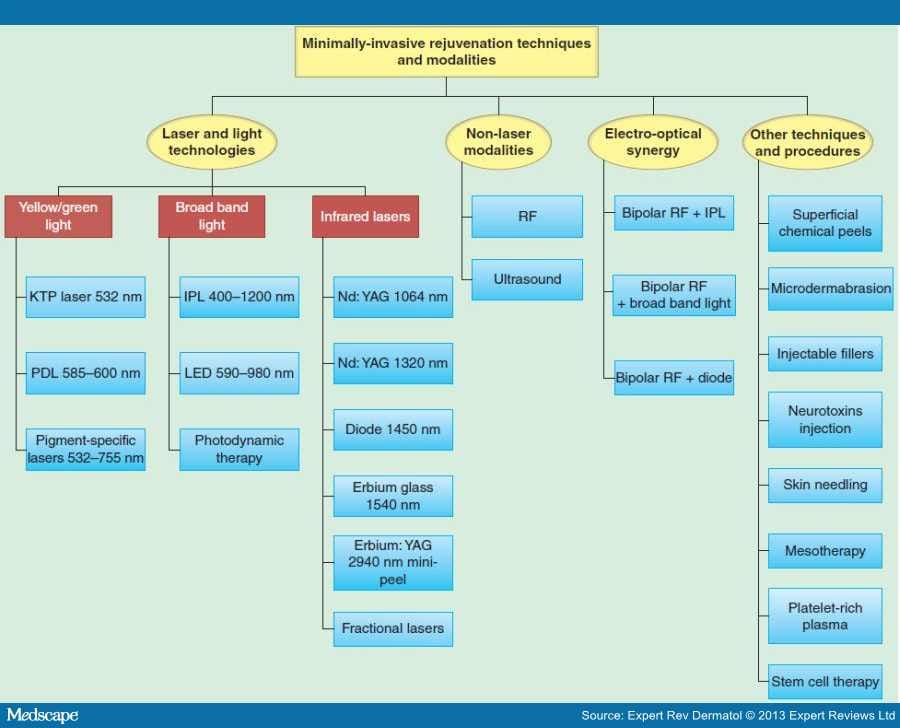
Figure 1.
Minimally invasive techniques and modalities for skin rejuvenation.
IPL: Intense pulsed light; KTP: Potassium titanyl phosphate; LED: Light-emitting diode; PDL: Pulsed dye lasers; RF: Radiofrequency.
Lasers and light sources used for non-ablative photorejuvenation could be classified based on their wavelengths into: i) yellow to green light, ii) systems emitting broad band light and iii) those emitting light in the infrared range (infrared lasers target pigment, hemoglobin and water).
Potassium Titanyl Phosphate Laser 532 nm. The potassium titanyl phosphate (KTP) laser uses a 1064 nm neodymium:yttrium-aluminum garnet (Nd:YAG) source passed through a KTP crystal to emit light with a wavelength of 532 nm. [26] This green light wavelength is absorbed by both hemoglobin and melanin. As a result, both unwanted vessels and pigment can be treated. At the same time, textural improvement is also seen, but to a much lesser extent. [18]
The KTP laser’s 532 nm wavelength corresponds with the 542 nm absorption peak of hemoglobin, which makes it relatively specific for cutaneous blood vessels. [26] Single vessels are traced by using a spot size close to the same vessel’s diameter. This will bring energy only to the targeted vessel and closely nearby tissues, leading to photocoagulation without extravasation of the vessel content, with subsequent sparing of normal capillaries. [27,28]
On comparing green with yellow light, the high absorption of 532 nm KTP by melanin is the only theoretical disadvantage, making it unsuitable for treatment of darker skin types. [29,30]
Pulsed Dye Laser. Pulsed dye lasers (PDL) emit yellow light at 585–595 nm which selectively targets hemoglobin and melanin. This wavelength permits a 50% dermal penetration with 400 μm depth, and is exclusively absorbed by blood vessels. Thus, enhancing the release of inflammatory mediators from endothelial cells within the targeted vessel with subsequent stimulation of fibroblast activity to produce new collagen. [24,31]
Many studies suggested the potential role of PDLs in the treatment of photodamaged skin by the clear clinical and histologic improvement seen with PDL-treated patients. [15] Improvement in the appearance of wrinkles has been observed following exposure to short pulsed 585 nm laser light at low energy levels (2–3 J/cm 2). [25] Longer pulses theoretically allow more heating of larger capillaries with less risk of purpura, thus reducing the downtime. [32]
Despite approval by the US FDA for treating photodamage with the PDL, only modest results have been observed with these wavelengths, presumably because of predominantly vascular targeting and superficial penetration to the papillary dermis. [15]
Pigment-specific Lasers. Pigment-specific lasers are used to treat the pigmentary changes that occur with photodamage, including solar lentigines, ephelides or freckles. These include Q-switched (QS) Nd:YAG (532 nm), QS ruby (694 nm) and QS alexandrite (755 nm) lasers as well as QS 1064 nm (infrared spectrum) laser. [8,24]
The frequency doubled Nd:YAG laser emits radiation with a wavelength at 532 nm and a pulse duration in nanoseconds. The use of QS lasers in the treatment of pigmented lesions follows the principle of selective photothermolysis thus limiting the damage to the melanosome-containing cells. However, at 532 nm, the wavelength is absorbed not only by melanin but also by hemoglobin. [24,33]
Ruby laser 694 nm with 28–50 ns pulse duration, was the first QS laser system produced for epidermal and dermal pigmented lesions; however, caution is a must as patients with darker skin types can develop permanent hypopigmentation. [33,34]
The QS alexandrite 755 nm laser with 50–100 ns (slightly longer pulse duration) is used to treat lentigines with less epidermal disruption. Whitening without ablation of the epidermis is usually the treatment end point. [35] Bruising, crusting as well as temporary and permanent pigmentary changes are not uncommon side effects, leading to some longer downtime. [33,36]
Intense Pulsed Light. As one of light-based technologies, intense pulsed light (IPL) is used to rejuvenate aging skin; it emits a non-coherent polychromatic light with filtered flashlamp source in a broadband wavelength (400–1200 nm) in the visible and mid-infrared ranges of the electromagnetic spectrum. Meanwhile, cutoff filters are used to allow a defined wavelength to penetrate the skin and target particular structures. [37,38] The device is capable of emitting yellow, red and infrared simultaneously so that multiple components of photoaging can be treated concurrently. [39,40]
Similar to lasers, IPL systems produce their effect based on the principle of selective photothermolysis. Unlike lasers, which treat one chromophore with monochromatic light, IPL systems have wide spectrum of probable combinations of wavelengths, pulse durations, pulse intervals and fluencies. IPLs have the ability to target both melanin and hemoglobin; thus treating vascular and pigmented lesions very efficiently with modest clinical improvement in wrinkles. [15,25]
IPL devices have the following advantages as they are safe effective treatments for redness or flushing of the face, neck and chest and they exert substantial visible improvement with no downtime, bruising or crusting. Disadvantages of IPL devices include their large spot sizes and bulky handpieces, making its application to small concave areas of the face difficult. Another disadvantage is the lack of real-time visibility of the treatment area due to the need of contact cooling for epidermal protection. [37,41]
Light Emitting Diode. Light-emitting diodes (LEDs) emit a narrow band of electromagnetic radiation, measured in milliwatts, ranging from the UV to the visible and infrared wavelengths. They can be classified as emitting wavelengths between lasers and broadband light. [42] An array of LED with dominant wavelength of 590–980 nm is used for treatment of photoaged skin. More specifically, they produce pulses of low energy, non-laser and non-thermal light that modulate the biologic activity of keratinocytes and fibroblasts by affecting the mitochondria, increasing collagen production. [18]
LEDs are typically assembled on small chips or equipped with tiny lenses put together into small lamps, LED is safe for all skin types and is fast and convenient to use. [31,39,42] Although the biological effects on skin cells seem to be evident (wound healing, reduction of chronic and acute actinic damage), clinical results of skin rejuvenation obtained simply with LED photomodulation are not particularly convincing either on skin tonus or on wrinkle reduction. [33,43]
Photodynamic Therapy. Photodynamic therapy (PDT) is defined as a photochemical reaction used to selectively destroy tissue. It is considered as a form of photochemotherapy that uses a photosensitizer, light and oxygen. [44] The use of PDT for skin rejuvenation has been well documented in different studies. It is a two-stage therapeutic technique in which the use of a topical (5-aminolevulinic acid (5-ALA; 20%) or methyl aminolevulinate [MAL]) or systemic (hematoporphyrin [Hp], hematoporphyrin derivative [HpD] or systemic 5-ALA) sensitizing drug, is followed by visible light radiation of appropriate wavelength (IPL, LEDs, PDL and blue light [410–490 nm]). [45]
The photosensitizers, administered exogenously or formed endogenously, are activated by the light and transfer energy to molecular oxygen, thereby generating reactive oxygen species to induce cell death. [46,47] Protoporphyrin IX has its largest absorption peak in the blue region at 410 nm with smaller absorption peaks at 505, 540, 580 and 630 nm. However, a blue fluorescent lamp (peak emission 417 nm) is used in Levulan-PDT. [48,49] The majority of clinical studies are performed using light wavelengths of 625–633 nm, which permit greater skin penetration. The effective therapeutic depth appears to be close to 1–3 mm when 635 nm is used. This is due to the capacity to produce a photodynamic reaction, which also depends on the dose of light and also on the quantity of photosensitizer used in the target tissue. [45,50]
Different studies suggest that PDT may improve the appearance of wrinkles and fine lines, telangiectasias, skin tone and photodamage. For the use of IPL in PDT, usually handpieces with a cut-off filter allowing transmission of light above 600 nm (used for hair removal) are suitable. Pulse duration can be set at a large range (millisecond). Short pulse duration plays a role particularly with respect to pain. [51] In comparison with continual irradiation with red light, PDT with a flash lamp is perceived as less painful. The various probable parameters of IPL with respect to wavelength, pulse duration, pulse interval and energy density make targeted use possible for the experienced dermatologist, on one hand, but make the comparison of different studies difficult on the other. [52,53]
Nd:YAG Laser, 1064 nm Long Pulsed & Short Pulsed Q-switched. The long pulsed Nd:YAG emits energy in infrared spectrum at 1064 nm with extended pulse duration. The laser results in diffuse heating of dermal tissue caused by the deeply penetrating nature of 1064 nm, which has an optical penetration depth of 5–10 mm. [39] The chromophores for the 1064 nm laser are, in decreasing order, melanin, hemoglobin and water. Water weakly absorbs laser energy at this wavelength and is gently heated; however, severe heating remains localized to hemoglobin and melanin. [24,43]
QS 1064 nm Nd:YAG is one of the first lasers used for non-ablative skin rejuvenation. Its long wavelength and ultra-short pulse duration of nanosecond, allow penetration of the papillary dermis with subsequent dermal wounding and limited thermal diffusion to the proposed target. Multiple treatments are required to attain best results. [30,54]
Facial telangiectasia (spider veins) and mild photodamaged skin are main clinical indications. [43,55] The QS 1064 nm Nd:YAG laser could be also used for treating pigmentary changes beside vascular changes that occur with photodamage, because it highly targets melanin within dermal melanocytes. [24,33] Meanwhile, side effects include mild erythema in all patients (lasting from 1 to 2 h), purpura, rarely post-inflammatory hyperpigmentation and temporary hypopigmentation, can be avoided by using lower fluencies. [31,55]
Nd:YAG 1320 nm Laser. The 1320 nm laser system was the first available system designed exclusively for selective dermal heating. The primary chromophore of the 1320 wavelength is dermal water; which is well scattered horizontally and vertically, thus allowing for maximal dermal injury. [18,22,31]
The Nd:YAG 1320 nm laser has beneficial effect in reversing the signs of skin aging at both the clinical and histological levels. [54,56–58] At the clinical level, Nd:YAG enhances skin tightening and reduces wrinkles. At the histological level, Nd:YAG enhances the formation of newly synthesized collagen and increases the dermal matrix contents with improving the morphologic appearance of collagen I and III together with elastic fibers. [58]
Diode Laser 1450 nm. The 1450 nm diode laser produces low peak powers (10–15 W); that means relatively long exposures are necessary in order to achieve sufficient fluences for selective dermal heating. These longer pulse durations require cooling to be delivered in a sequence of sprays before, during and after the pulse. [59]
In the infrared portion of electromagnetic spectrum (>700 nm), the water absorption coefficient is relatively low, [60] allowing this infrared technology to target tissue water and penetrates skin to a depth of approximately 500 μm. It is used for facial rejuvenation targeting the water in the upper dermis, this laser remodels the skin’s underlying collagen and promotes formation of new collagen, improving facial and periorbital rhytides. Patient acceptance of the treatment was high, but most felt that there was little improvement of the treated rhytides. [18,39] Side effects are usually minimal and can include postoperative erythema, edema and hyperpigmentation in patients with darker skin type. [24,60]
Erbium: Glass Laser 1540 nm. The erbium:glass 1540 nm laser is a flash lamp-pumped system with yttrium-erbium phosphate glass. Similar to other infrared lasers, its wavelength is highly absorbed by water but minimally by melanin. The wavelength is delivered in 10–100 ms pulses with fluences ranging from 20 to 30 J/cm 2. [39,61] The skin is cooled using a handpiece; which comes in direct contact with the skin, with purified tetrafluoroethane cryogen circulating inside. The handpiece has a real-time temperature monitor at the sapphire for immediate feedback. [62]
The primary depth is within the papillary dermis where collagen tightening and neocollagenesis are achieved. The erbium:glass 1540 nm laser is used to treat a variety of conditions through the destruction of the sebaceous glands by warming up of the tissue and reducing sebum production. It is also used in skin rejuvenation, scars and acne scars by stimulating the formation of new collagen. This leads to an improvement of the skin structure as well as a reduction of wrinkles and pore size. [31,63]
Advantages of therapy include a lack of pain, discomfort or downtime. Disadvantage of therapy is that patients may have great expectations and may be disappointed with the results. Improvement is slow (occurring in months) and mild, with most patients appreciating more elastic and firmer skin. [31]
Er:YAG 2940 nm Mini-peel. Although it is utilized primarily for ablative resurfacing, erbium:yttrium-aluminum garnet (Er:YAG) laser has been used for non-ablative rejuvenation. [24,64,65] The Er:YAG laser is characterized by high absorption coefficient of its mid-infrared radiation in water; thus inducing minimal thermal injury to the underlying tissue. [66] The zone of residual thermal damage (RTD) is typically 20 ± 50 μm deep; which results in faster skin re-epithelialization. [67] This started the idea to produce deep collagen denaturation by stacking of repetitive Er:YAG pulses. [68–71]
Micro-resurfacing is a technique that employs the use of Er:YAG laser system to deliver a single-pass ‘mini-peel’. The use of a sequence of short Er:YAG pulses (200–270 ms) below the ablation threshold increases the temperature in the upper dermis to about 60°C in order to induce collagen denaturation. [72,73] Benefits of this technique include that it is an effective, well-tolerated and minimally invasive treatment option for photoaging as it stimulates collagen formation and remodeling of extracellular matrix (ECM) proteins without ablation of the epidermis. This is accompanied by a noticeable clinical improvement of wrinkles and photoaged skin with the advantage of minimal downtime and side effects. [64,65]
Multiple passes over the wrinkles result in a thermal build up by heat conduction. As a result, the optical penetration depth is increased; resulting in further diminished ablation efficiency, enhanced deposition of heat and increased zone of thermal injury. [64,74,75]
A recent study by El-Domyati et al. showed the effect of multiple passes using Er:YAG 2940 nm laser mini-peel on subjects who were treated on the face every 2 weeks for 3 months for a total of six sessions. [65] A moderate clinical improvement (Figure 2), accompanied with significant histologic findings in the form of increased types I and III collagen and decreased dermal elastin in response to treatment was reported.
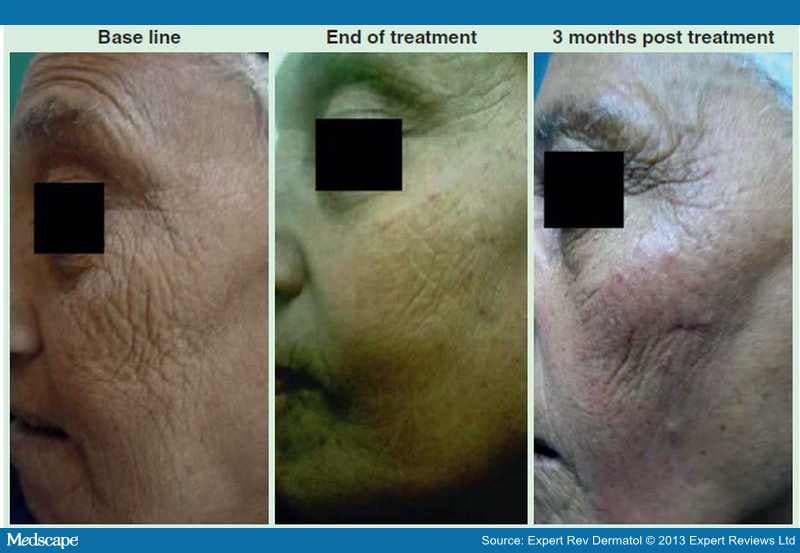
Figure 2.
Representative photographs of a patient treated with Er:YAG 2940 nm laser mini-peel showing moderate improvement of wrinkles in response to treatment.
Fractional Lasers. Fractional photothermolysis is a novel technology for skin rejuvenation that can be considered intermediary between ablative and non-ablative resurfacing; it could be achieved with non-ablative and ablative modalities. [76]
True non-ablative fractional laser requires three criteria: i) non-ablative mode of tissue coagulation, with preserving the stratum corneum, ii) creation of multiple microthermal zones (MTZs) surrounded by islands of viable tissue and iii) resurfacing with extrusion and replacement of damaged tissue, with re-epithelialization within 24 h. [77,78] With this technology, fractional lasers are employed creating thousands of tiny treatment zones on the skin, microscopic columns of thermal injury (microthermal zones), the depth of penetration ranges from 300 to 700 μm based on fluencies. [77,79,80] The target chromophore for the fractional laser is water; however, treatment is performed in a pixilated fashion, leaving approximately 70% of the skin undamaged to promote rapid healing. The wound-healing response differs from that of other techniques because viable cells exist between treatment zones, including epidermal stem cells and transient amplifying cell populations.[31] Each laser hit produces a 30–70 μm plug of microscopic epidermal necrotic debris that naturally exfoliates in approximately 14 days. [81]
Relative epidermal and follicular structure sparing is responsible for rapid recovery without prolonged downtime. Melanin is not at risk of selective targeted destruction; therefore, fractional resurfacing has been used successfully in patients with dark skin color. [57,76] Dermal effects of microthermal zone repair generate wound mediators that ultimately lead to remodeling of the dermal matrix and histologic demonstration of enhanced rete ridge which enhance skin rejuvenation. [76,82]
Ablative fractional modalities are laser systems using an ablative laser (Er:YAG or carbon dioxide) pulse that only hits a fraction of the skin at each pulse. This allows many skip areas in between the MTZs to quickly re-epithelialize the wounded skin. MTZs allow delivery of high local irradiance to achieve efficacy while maintaining low overall irradiance to prevent side effects. [82] Unlike non-ablative fractional photothermolysis, these devices cause true ablation of the epidermis in addition to variable depths of ablative damage to the dermis. The combination of epidermal and dermal ablation appears to lead to a more robust wound-healing response and accompanying dermal fibrosis, which may explain the rapid and significant clinical effects that can be achieved with ablative versus non-ablative devices. [83]
Complications with non-ablative fractional laser resurfacing are rare and generally self-limiting. Prolonged erythema has been reported with higher fluencies, but generally resolves. Microthermal zone pattern persistence can occur and usually resolves within 2–3 weeks. [84]
Radiofrequency. RF energy has been used for more than a century in a variety of medical applications. Non-ablative RF (monopolar, bipolar, tripolar or multipolar and fractional) was described by different studies as an effective and safe approach for skin rejuvenation. [3,85] Depending on the delivery system and frequency, superficial or deep heating can be produced.[18,86] RF was approved by the FDA in 2002 for the non-ablative treatment of wrinkles and skin tightening and for full-face treatment in 2004. [87,88]
Essentially, RF devices consist of a RF generator, automatic resistance test technology via computerized software, continuous cooling system, hand piece and different size treatment tips. [39,78] The RF generator produces alternating current that creates an electric field through the skin. The electric field shifts the polarity millions of times per second, causing a change in the orientation of charged particles within the electric field. Thus, heat is generated by the skin’s resistance to the flow of current within an electric field. [57,89,90]
The mechanism of action of RF is dual in nature: i) an early instant collagen contraction and ii) a secondary wound-healing response, in the form of new collagen formation and remodeling with eventually tightening. The immediate skin contraction is attributed to the sparse pattern of collagen denaturation, while sufficient tissue is left healthy to ensure wound healing. [3,91]Over time, as a thermally mediated healing response, heated fibroblast stimulation enhances formation of new collagen leading to further collagen tightening and overall increase in collagen deposition. [92] Additionally, another mechanism of action for monopolar RF has been based on the fact that the delivered energy usually favors the least resistance path. As subcutaneous fat lobules are divided by interweaving network of collagen-based septa, these fibrous septa are preferentially heated resulting in collagen tightening. [93,94] This gives the patient immediate, visible improvement the day of the procedure with subsequent lifting and remodeling of subcutaneous tissue, as well as the skin becomes tightly attached to the underlying structures. [94,95]
Unlike most lasers, which target specific chromophores, the output energy of the RF is a chromophore-independent; it does not follow the principles of selective photothermolysis. Heat is generated as a result of tissue resistance to the movement of electrons within the RF field; [90] allowing energy to be delivered to 3D levels of the dermis. [16,90]
El-Domyati et al. analyzed the effect of monopolar RF on individuals who underwent treatment on the face every 2 weeks for 3 months for a total of six sessions. [3] Punch biopsies of the facial skin were performed at baseline, end of treatment and 3 months after treatment. Noticeable clinical improvement (Figure 3), together with significant histologic findings including decreased dermal elastin as well as increased collagen types I and III was observed after treatment.
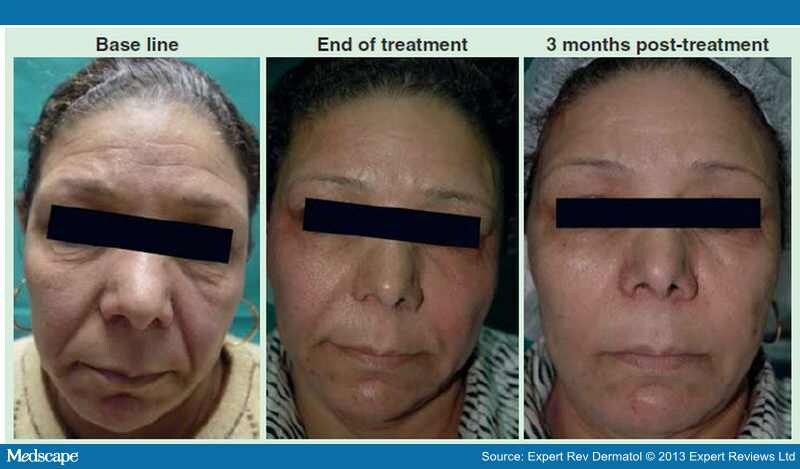
Figure 3.
Clinical response to monopolar radiofrequency treatment showing improvement of forehead, periorbital and nasolabial areas at end of treatment with continued improvement at 3 months post-treatment, compared with base line.
Bipolar RF devices pass electrical current only between two positioned electrodes applied to the skin. No grounding pad is necessary with these systems, as monopolar RF, because no current flows throughout the remainder of the body. It is claimed, however, that bipolar RF cannot produce a uniform volumetric heating comparable at all with monopolar RF. [37,91,96]
The use of a tripolar (multipolar) device has been explored for the treatment of skin aging; the device has three probes that exploit the benefits of both unipolar and bipolar RF to treat tissue. Although bipolar devices rely on active integrated cooling devices to avoid damage to the epidermis, the tripolar device advertises simultaneous moderate deep and superficial heating of tissues that does not require protective cooling for the epidermis. [97]
Newer RF technologies including devices with multigenerational sources and fractional RF system have emerged with more promising potential since then. Fractional RF has the non-invasive modes of known RF devices with the development of a minimally invasive bipolar microneedle delivery system. It generates localized coagulation zones within the reticular dermis characterized as RF thermal zones. This fractional RF system offers controlled dermal heating through pulse duration variance, allowing for fractional sparing of the epidermis and important adnexal structures. [78,96] Studies showed that the treatment-generated RF thermal zone in the reticular dermis consists of denatured collagen separated by spared dermis. These zones were replaced by new dermal tissue within 10 weeks. [96]
Ultrasound. Ultrasound devices were first approved for eyebrow lifting in the USA in 2009, and have subsequently been used for treatment of skin and tissue laxity. [98] This modality uses ultrasonic energy to produce specific micro-coagulation zones deep in the dermis and subcutaneous adipose tissue. During the months following treatment, repair of the deep tissue damage leads to contraction and tissue remodeling, resulting in the desired aesthetic effect of reduced skin laxity. [99,100] The superficial dermis and collateral tissues are spared, which not only decreases the risk of scarring and downtime but potentially permits to be used in different skin types. [98–101]
ELOS, combined electrical and optical energy, is a new technology which has been recently introduced as a non-ablative treatment for skin rejuvenation. This technology uses RF and optical energy from laser or light sources within the same device to be combined in the same pulse profile. It is based on the principle of synergistic activity between the two forms of energy. [10,90,102]
The ELOS system consists of a bipolar RF generator and a flashlamp. The pulsed light is delivered through a contact sapphire light guide with the bipolar RF energy which is delivered through electrodes embedded in the system applicator and brought into contact with the skin surface. The temperature of the tissue is continuously measured throughout the duration of the pulse to prevent overheating and improve safety. Some devices use non-laser light source (580–980 nm) for optical energy, whereas others use a high-power diode laser (900 nm) as its light source. [102–104]
The mechanism of this new technology is based on two combined approaches: the first approach includes light-based technologies where the pulsed optical energy targets and preheats distinct chromophores producing differences in temperature between the target and the surrounding tissue, according to the principle of selective photothermolysis. [10,105]The second approach uses the creation of stress waves at the skin surface by RF inducing uniform heat at controlled depth to dermal layers. The RF energy usually follows the least resistance path, a phenomenon known as impedance. [10,16]
The generated heat by pulsed optical energy will reduce the target impedance providing favored conduction pathway for the RF toward the target of interest. [106] As a result, the combined forms of energy act synergistically to initiate thermal wound at the targeted area with subsequent remodeling and reorientation of collagen bundles and formation of new collagen; which are achieved over months after treatment. [90,94,107]
A recent study by El-Domyati et al. evaluated the histologic changes and corresponding clinical outcomes after use of the ELOS technique (RF combined with IPL) for skin rejuvenation. [10] The volunteers were treated for a total of six sessions at 2-week intervals. The outcomes were assessed using photographs and punch biopsies taken at the end of the treatment and 3 months post-treatment, that showed improvements in skin tightening, texture and wrinkles, which was accompanied by reduction in elastin 3 months after treatment and a significant increase in collagen types I and III (Figure 4).
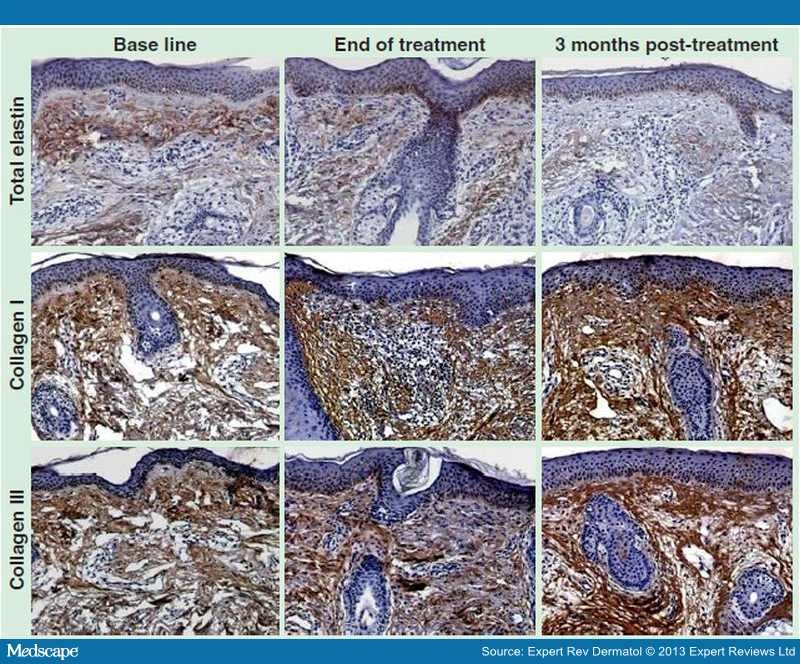
Figure 4.
Dermal elastin and collagen contents in response to electro-optical synergy treatment. Immunoperoxidase staining of skin tissues at base line (left panels), end of treatment (middle panels) and 3 months post-electro-optical synergy treatment (ELOS) treatment (right panels), for total elastin (top row) and collagen types I and III (middle and bottom rows); showing a decrease in dermal elastin upon ELOS treatment (top row) with an increase in collagen type I (middle row) and type III (bottom row) contents in response to ELOS (immunoperoxidase, x200 magnification).
The bipolar RF component enables the use of lower levels of the optical component, reducing the risk of optical energy and potentially improving its use across different skin types. The optical component is believed to drive the bipolar RF energy to concentrate where the optical energy has selectively heated the target. [102,108] This technology appears to be an ideal option for darker skin types due to the fact that optical energy has weak absorption of melanin and RF energy does not depend on chromophores for its effects. [22] It can be used to rejuvenate the aged skin and reverse the signs of photoaging safely and effectively. This modality stimulates the remodeling process, and improves the clinical and histological signs of aging, with the advantage of being a safe procedure and avoiding significant downtime. [10]
Side effects associated with combined electro-optical energy are uncommon. [104,109] The use of lower energy levels allows patients to tolerate the procedure well with minimal discomfort and no need for adjunctive anesthesia. The most common side effect is transient erythema immediately after the procedure, usually resolving in minutes to hours. Crusting, blisters, pigmentary change and scarring are rare. [90,108]
Chemical Peeling. Chemical peeling is the topical application of chemical agents to the skin, which causes controlled destruction of part or the entire epidermis, with or without the dermis, leading to exfoliation and removal of superficial lesions, followed by regeneration of new epidermal and dermal tissues. [7]
Chemical peels can be classified into: i) very superficial (exfoliation) which includes destruction of the stratum corneum without creating a wound below the stratum granulosum, ii) superficial (epidermal) including destruction of part or all of the epidermis, anywhere from the stratum granulosum to the basal cell layer, iii) medium (papillary dermal) with destruction of the epidermis and part or all of the papillary dermis and iv) deep (reticular dermal) which includes removal of the epidermis and papillary dermis, extending into the reticular dermis. [110,111]
Superficial chemical peels are currently performed with various compounds including trichloroacetic acid (TCA), alpha hydroxy acid (glycolic acid) and Jessner’s solution (14% lactic acid, 14% resorcinol and 14% salicylic acid in ethanol). [17] TCA produces superficial peeling (minimally invasive) when used in dilutions from 10 to 35%. [112,113]
Indications for chemical peeling include pigmentary disorders, acne and superficial acne scars, skin aging and benign epidermal growths. Relative contraindications may include dark skin type of the patient and patient with unrealistic expectations. [114] Meanwhile, absolute contraindications include active bacterial, viral or fungal infection, open wounds, pre-existing inflammatory dermatoses, non-cooperative patient (regarding sun exposure or application of sun block) and history of abnormal scarring, keloids, atrophic skin or isotretinoin use in the last 6 months. [86]
Microdermabrasion. The concept of facial resurfacing was reported as early as 1500 BC, when the Egyptians used sandpaper to smooth scars. Dermabrasion, the technique that penetrates to the depth of the dermis to promote skin regrowth and rejuvenation, was developed in the early 1900s and modified to its modern form in the 1950s. [115,116]
Microdermabrasion or particle resurfacing was designed in the 1980s and gained popularity because of its ease of use, relatively benign nature and its proposed gross effectiveness. It is a minimally invasive procedure that relies on an abrasive component and a vacuum tube causing mechanical removal of the superficial epidermis and stimulation of new cell growth.[117]
Different microdermabrasion systems are known based on the source of the abrasive component. Most systems use inert crystals usually aluminum oxide, sodium chloride or sodium bicarbonate crystals that is propelled to the skin surface using a handpiece. In other systems, the coarse stimulus is a handpiece with rough crystals (diamonds); these crystals are fixed to the surface of handpiece which comes in direct contact with the skin. [118,119]
Vacuum suction is concurrently used to gather worn-out crystals and debris which are caused by the crystals’ abrasive action and transferred via a separate set of collection tubing into a dissipate container. [120,121]
Several authors have illustrated that microdermabrasion may play a role in the improvement of skin contour irregularities, including rhytides via mechanical removal of the stratum corneum and stimulation of new cell growth. Although microdermabrasion can be used to improve certain pigmentary changes, it might be less effective when compared with chemical peels. [111,122,123]
The side effects associated with microdermabrasion are minimal, the majority of patients may develop erythema and mild pain, meanwhile minor abrasions and petechiae may occur if the procedure is carried out aggressively. Yet, they are usually short-lived, lasting about 1 week. [117,119] Microdermabrasion is considered to be safe in all skin types, and the risk of post-inflammatory pigmentary changes is minimal. [111]
Injectable Fillers. Dermal fillers are one of the most common and useful treatments for wrinkles and folds. Over the past few years, there have been tremendous advances in the use of fillers. They have provided enhanced results as well as longevity for treating folds, wrinkles, pitted scars and skin depressions of the face bringing back its fullness. [124,125]
Fillers can be classified according to their permanence in tissues into: permanent, semipermanent and temporary. The majority of injectable fillers are temporary, lasting from several weeks (short lived) to several months (long acting); which require ongoing treatment to maintain the desired appearance. [126,127] Permanent fillers remain in the site of inoculation for years, but they are no longer suggested to be used, as their long-lasting safety has not been established; on the other hand, some other studies have revealed outstanding results for the use of these permanent fillers. [128]
Temporary fillers contain different substances including collagen and hyaluronic acid (HA) which is the most commonly used material. HA polysaccharide is a natural component of human dermis and epidermis, consequently, HA-based fillers have exceptional biocompatibility while providing indistinguishable structural as well as mechanical properties of normal human subcutaneous tissue. Hyaluronan, a naturally occurring substance, is rapidly broken down by hyaluronidase enzyme and metabolized to carbon dioxide and water after a short half-life of about 12 h. Thus, cross-linking increases HA fillers ( in vivo) lifespan from 6 to 18 months. [126] Factors that impact HA persistence include HA concentration, percentage and type of cross-linkage, water binding capacity and injection technique. [128]
Some filler substances such as polylactic acid or hydroxylapatite have long half-life. Fillers composed of polyacrylamide hydrogel and polyalkylamide remain permanently in the soft tissues, as well as liquid silicon. Although the subcutaneous injection of liquid silicone was declared illegal in many countries, it is still in use because of the low cost compared with other fillers. [129]
Minimally invasive dermal fillers used alone or in combination with botulinum toxin (BTX), have accounted for much of the surge in non-surgical facial rejuvenation procedures. [124] For motion wrinkles (dynamic lines caused by facial movements), the optimal tactics in many cases is to try BTX first. In fact, even if BTX has failed, it may still be a good adjunct to filler because simply injecting filler does not eliminate the cause of a motion wrinkle. [129–132]
Although fillers are generally regarded as safe, adverse outcomes can occur with these agents. Superficial placement of dermal fillers is a common error and is associated with a range of complications, from obviously visible product to inflammatory nodule formation and even hypertrophic scarring. Furthermore, complications may include unrealistic patient expectations, bruising/hematoma, undercorrection, overcorrection, asymmetry, lumping, granuloma and iatrogenic as well as allergic reaction. [124]
Botulinum Toxin. BTX is an injectable medication that relaxes the overactive contracting muscles to treat wrinkles mainly in the upper face. It was discovered as a toxin created by the Clostridium botulinum bacterium that causes food poisoning and muscle weakness; this toxin inhibits the release of acetylcholine from neuromuscular presynaptic vesicles producing a temporary muscle paralysis. [133,134] Thus, the muscles cannot contract to enhance existing wrinkles or to encourage new ones. This results in a much smoother, younger-looking appearance for the face. [134,135]
There are seven distinct serotypes of BTX: A, B, C1, D, E, F and G. Serotypes A and B (BTX-A and BTX-B) are available commercially. Botulinum toxin A (BTX-A) has successfully been used in the treatment of hyperkinetic facial rhytides since the late 1980s, while BTX-B is less commonly used and data regarding the efficacy and optimum doses are few. [110]
Tiny amounts of BTX are injected directly into the muscle that is causing wrinkles or dynamic lines. These injections are adjusted in doses of International Units (IU). [135,136] BTX injections are safe when done properly by an experienced and licensed physician who is an expert in facial anti-aging therapy and aware of muscle anatomy. However, there are potential side effects which can occur, some are unavoidable and others are related to injection technique. The most common potential sequelae of BTX are: overcorrection, undercorrection, asymmetric result, upper eyelid ptosis, neck weakness, perioral droop, bruising, intravascular injection, diplopia (lateral rectus) and psychosomatic problems. [130,133,137]
Microneedling Therapy. Microneedling therapy, also known as percutaneous collagen induction (PCI) therapy, is a recent addition to the treatment armamentarium for skin rejuvenation. The treatment is performed as an office procedure after application of a local anesthetic cream, by means of an instrument known as a dermaroller. [138]
A dermaroller is a simple, hand-held instrument consisting of a handle with a cylinder studded all around with fine, stainless steel needles 0.5–2 mm in length. This needle-studded cylinder is rolled on the skin in multiple directions to achieve a therapeutic benefit and hence the name ‘dermaroller’. [139]
During treatment, the needles pierce the stratum corneum and create microconduits (holes) without damaging the epidermis. It has been shown that rolling with a dermaroller (192 needles, 200 μm length and 70 μm diameter) over an area for 15-times will result in approximately 250 holes/cm 2. Microneedling leads to the release of growth factors which stimulate the formation of new collagen (natural collagen) and elastin in the papillary dermis. [115,116]
Treatment with dermaroller is performed at 4- to 8-week intervals and multiple sessions are needed to achieve the desired effect on the skin. Microneedling or dermaroller treatment is becoming popular all over the world; the treatment can be performed in an office setting and does not need any extensive special training or expensive instruments. [139–141]
Although dermarollers are easy to use, yet there are some disadvantages such as inability to treat small areas or localized scars as the roller damages adjacent skin too. In addition, the pressure used for rolling insults the epidermis. All these difficulties led to introduction of dermastamps as well as automated microneedling devices to the market. It is a pen-like instrument with handle, disposable needles and guides (to adjust needle length). The needle tip is having 9–12 needles arranged in rows. It has different modes of speed and controlled depth. Automated microneedling devices are easy to use as the user has to just put the device in stamping action on skin and the perpendicular movement of needle leads to penetration at required depth. [142,143]
Biorejuvenation (Mesotherapy). Biorejuvenation, also called biorevitalization, is a common term of mesotherapy for skin rejuvenation. Mesotherapy is a non-surgical cosmetic medical treatment belonging to homeopathic medicine which is usually administered by alternative medical practitioners. It does not indicate a treatment of any condition in particular; it simply describes a method of drug delivery. [144,145] Mesotherapy, originally used in Europe, based from Greek word ‘mesos’ which means middle, and ‘therapeia’ that means to treat medically, has been one of the newest techniques in cosmetic medicine to rejuvenate the aging skin, and it is performed by medical and non-medical professionals. [146,147]
Mesotherapy is a minimally invasive technique that consists of intradermal injection of variable mixtures of natural plant extracts, homeopathic agents, pharmaceuticals, vitamins and other bioactive substances in microscopic quantities through multiple dermal punctures. [148] It has been used to rejuvenate (mesoglow) and eventually tone (mesolift) the injected areas of the face and other body areas like neck, low neckline (decoltage), dorsum of hands, belly and inner surface of arms and legs. The most common and simplest formulation of mesotherapy for facial skin rejuvenation involves injection of a multivitamin solution into the dermis over the course of multiple sessions. [146,149,150]
It has been claimed that intradermal vitamin injections rejuvenate the cells, making them more active and thereby stimulate the production of collagen and elastin through increasing the biosynthetic capacity of fibroblasts. Injection of hyaluronic acid is supposed to promote skin rejuvenation by increasing hydration and recreating a favorable environment to facilitate fibroblast activation and interactions between cells and ECM. [144]
There are no published clinical data on standardized reagents, treatment protocols (including dose/injection, technique/injection and interval times) or appropriate positive and negative controls and end points. Although the US FDA has approved most of the mesotherapy ingredients used in injection, the components are being applied for unapproved indications. [146,149,151] Usually, treatments are given initially as once per week for 4 weeks, once every 2 weeks for 2 months and then once per month to achieve best results. [148,152]
The performed injection techniques can be one of the following: i) intra-epidermal technique involves placing small quantities of the medicine within the epidermis. It is simple, painless and there is no bleeding, ii) papular technique involves injecting the medicine at the dermoepidermal junction, iii) Nappage technique, in this approach injections are given at a depth of 2–4 mm at an angle of 30–60° and iv) point-by-point; this is a precise single injection into the deep dermis. [147,151,152]
The controversy surrounding mesotherapy efficacy and potential adverse effects are still of concern to many researchers.[152] As with any new procedure, it is important to assess the benefits, safety, efficacy and standardization before mesotherapy could be advocated for the treatment of skin rejuvenation. [145]
Although it is an easy method to perform, side effects depend on the product used; bruising and edema are common due to the inflammatory response provoked by some of the chemicals used in mesotherapy. Following mesotherapy, atypical mycobacterial infections have been reported at sites of injections necessitating antimycobacterial therapy. [146,149–151]
Platelet-rich Plasma. PRP is an autologous preparation of platelets in concentrated plasma. PRP is being widely applied in various medical fields including dermatology for its ability to stimulate wound healing. Further, it has been used clinically in mesotherapy for skin rejuvenation. [153] Although PRP injection is considered one of the minimally invasive procedures, it usually causes skin bruises which will result in longer downtime when compared with other minimally invasive modalities.
The α-granules of concentrated platelets secrete a variety of growth factors after being activated by aggregation enhancers. These factors including VEGF, PDGF IGF and TGF are known to control cell migration, attachment, proliferation as well as cell differentiation with improving the production of ECM protein by binding to certain cell surface receptors. [154,155]
Since these growth factors are present in high concentrations, PRP has been used in different cosmetic surgeries as well as clinical treatments including challenging surgical wounds. [156] As PRP secretes a variety of growth factors in order to regenerate the skin, it may be hypothesized that PRP may promote new collagen synthesis as well as other ECM components through activation of fibroblasts; accordingly it is used to rejuvenate photoaged facial skin with consequential improving in its clinical appearance. [157,158] The secretion of various growth factors is triggered by the activation of platelets after its coagulation resulting in enhancing the mitogenic effects in different cell types. [159,160]
Up to date, there is no evidently clear method for the clinical application of PRP. Different methods are being tested including topical application or direct injection into the skin. The use of microneedling and lasers are another approach for enhancing skin remodeling by increasing penetration and producing mild inflammatory reactions. Meanwhile, the evidence-based anti-aging effects of this modality in vivo remain to be determined. Further studies are essential to conclude whether such procedures produce beneficial effects in aged skin. [153,161]
Stem Cell Therapy & Stem Cell Factors. The use of body’s stem cells and growth factors is another therapeutic modality for repair of damaged tissue, and cell-based therapy. The study of stem cells in dermatology is a rapidly emerging field in both basic and clinical research. [162]
Tissue-derived or adipose-derived stem cells (ADSCs) display multilineage developmental plasticity and secrete various growth factors, similar to fibroblasts action, such as IGF, VEGF and transforming growth factor-beta1 (TGF-β1), thus it was hypothesized that ADSCs may improve photodamaged skin. [163] Tissue-derived stem cells and its secretory factors have been shown to protect dermal fibroblasts from oxidative stress caused by ultraviolet radiation and chemicals. Studies also showed that the injection of ADSCs and adipose-derived stem cell conditioned media (ADSC-CM) stimulate migration of dermal fibroblasts with subsequent collagen synthesis during wound healing and reduce wrinkles. [44,164]
Advantage of this modality is the demonstrated safety and effectiveness of stem cells in repairing damaged tissue, because the pathophysiology of photoaging is similar to that of chronic wounds. In addition, ADSC-CM has been shown to increase protein expression of type I collagen and reduce the protein level of matrix metalloproteinases (which degrade collagen) in fibroblasts. [165,166]
The armamentarium offered for minimally invasive facial rejuvenation is strikingly expanding, however because each person is different, there is no one modality that is suitable for everybody. Thus, it is important not only to correct the wrinkles, but also to develop a cosmetic overall concept with the patient. This suggests that the choice of any of the treatment options should be selected according to the patient’s condition, needs and goals.
BTX injection may be useful for wrinkles associated with mild and early degrees of photodamage in young patients with hyperkinetic muscles, while lasers, light sources and RF technologies are more beneficial for more photodamage caused by loss of collagen. Meanwhile, combination therapy is highly useful for those having moderate to severe photodamaged skin with hyperkinetic muscles or volume loss. For appropriate reversal of signs of aging face, selection of a suitable combination should be put in mind. A combination treatment is considered an approach that includes at least two different and unrelated modalities, such as a light or laser device combined with non-laser technology, ELOS or other procedures or techniques. Often, appropriate patient selection and combination of different techniques allow individualized treatment with optimal outcomes.
Multiple sessions and combined minimally invasive modalities, beside the use of future home devices would fill the gap between ablative and non-ablative approaches in improving the signs of skin aging and maintaining the clinical and the histological improvement. Advanced cellular and skin aging knowledge and research paves the way for the technical evolution of the use of stem cells in skin rejuvenation. Meanwhile, advances of laser sources and techniques, fractional laser and other energy approaches, beside new and safer filler options, as well as new neurotoxin complexes represents a promising future for minimally invasive skin rejuvenation and treatment of the aging skin.
Papers of special note have been highlighted as:
* of interest
** of considerable interest
Expert Rev Dermatol. 2013;8(5):565-580. © 2013 Expert Reviews Ltd.
Facial exercises have been touted as a less invasive and less expensive alternative to traditional facial rejuvenation procedures, but do they really work? To evaluate the efficacy of this nonmedical approach to facial revitalization, researchers from Belgium systematically reviewed the medical literature, identifying nine studies that examined the effects of facial muscle exercises on facial rejuvenation. Although the authors of all studies reported positive outcomes, the research team found that the quality of the available evidence was insufficient for determining the efficacy of facial exercises for aesthetic rejuvenation. Their complete findings are published in a new article, “The Effectiveness of Facial Exercises for Facial Rejuvenation,” which appears in the January issue of the Aesthetic Surgery Journal.
“Our review shows that there is not enough evidence to conclude whether facial exercises are effective for reducing the signs of aging,” said lead author John Van Borsel, PhD, Professor of Neurolinguistics and Logopedics at Ghent University, Ghent, Belgium, and Veiga de Almeida University, Rio de Janeiro, Brazil. “The existing published studies were not randomized or controlled. Most lacked blinding and only used subjective measures to assess the effectiveness of treatment. We need better studies before we can draw any conclusions about the usefulness of facial exercises.”
Dr. Van Borsel and colleagues identified nine published studies, all from studies conducted in South America. None were randomized or controlled; instead, they were single case reports, small case series, or studies that used a single-group, pretest-posttest design. Nearly every study included more than one type of exercise and most used subjective assessments (i.e., assessment by the authors and/or patients). The researchers concluded that additional studies with superior designs and larger patient populations are needed—especially randomized, controlled, blinded studies evaluating a single type of exercise using objective measurements. They also noted that comparisons of different types of facial exercises are needed, as is information on the role of intensity and duration of treatment and on the effect of patient-specific variables such as age and signs of aging at onset.
“This is the first systematic review to look at the effectiveness of facial exercises for facial rejuvenation, and shows that we are really lacking evidence when it comes to the claims that facial exercises can rejuvenate the face,” said Foad Nahai, MD, Editor-in-Chief of Aesthetic Surgery Journal. “Randomized, controlled studies are the gold standard for determining the efficacy of any procedure. Hopefully, we will see some well designed studies in the future that can help us determine whether these claims have merit.”
According to the American Society for Aesthetic Plastic Surgery (ASAPS), 119,006 facelifts were performed in 2012 and nonsurgical facial rejuvenation procedures were highly popular: botulinum toxin type A injection (3,257,913 procedures), hyaluronic acid injection (1,423,705), microdermabrasion (498,821), and chemical peel (443,824).
November 23, 2013 12:54 pm by Melissa Dribben | 1 Comments
As legalized medical marijuana gains acceptance across the country, a long-smoldering question burns a little hotter.
In the vernacular, stoners ask, “Do doobies make boobies?” Plastic surgeons phrase it more scientifically. “Does marijuana cause gynecomastia?”
Speculation that men who smoke pot are prone to develop abnormal breast tissue or “man boobs” has been around for decades. The first scientific paper examining the clinical impact of the drug’s active ingredient, THC, on hormonal systems was published in 1972 in the New England Journal of Medicine.
This was about the same time Brewer & Shipley stoked Spiro Agnew’s ire with the hit single “One Toke Over the Line.” The drug’s ability to stoke controversy has not abated. The 1972 study found that the drug has “widespread effects on multiple hormonal systems, including gonadal, adrenal, prolactin, growth hormone, and thyroid hormone regulation.” When the drug throws off the normal balance of hormones, estrogen levels rise and stimulate breast tissue growth.
Subsequent studies have been few and their findings conflicting. As a result, marijuana’s advocates call the association a myth, asserting that there is no solid scientific proof.
They have a point, says Adrian Lo, a plastic surgeon at Pennsylvania Hospital who specializes in breast reduction for men. Because marijuana is illegal in most states, he explains, it’s hard to conduct research. But this does not make the link a myth.
“What we’re left with are doctors, endocrinologists, and surgeons with clinical acumen saying we notice a trend,” he says. Of the 100 or so patients who come to him for breast reduction surgeries each year, more than one-third report regular marijuana use.
“Some men are more susceptible to gynecomastia than others,” he says. Smoking pot can lower testosterone levels for 24 hours, he says. After just one joint, patients have reported feeling swelling and puffiness around the nipple, while regular users may have no reaction, at least in their breast tissue.
“We can’t predict who it’s going to happen to,” Lo says.
“I wouldn’t say I was smoking seven days a week, but it was close,” says a 23-year-old patient who recently underwent breast reduction. Worried about the legal ramifications and his job security as an actuary, he agreed to speak identified only by his middle name, Michael.
He first developed enlarged breasts when he was going through puberty, a few years before he started smoking. His mother took him to the pediatrician, who said the condition was normal and temporary. But Michael was among the small percentage who did not grow out of it.
For years, he would avoid baring his chest. “In games where the choice was shirts vs. skins, you never wanted to be skins,” he says.
Once he started having relationships with women, he worried about how they judged his body. At 5-foot-11 and 190 pounds, he was not overweight, and worked out three or four times a week at the gym. “I benched and lifted,” he says. “Underneath, I had pecs.” But no amount of exercise made his breasts turn to muscle.
He had heard that pot could cause man boobs. “When I was high, they felt a little more noticeable,” Michael says. “But I don’t know if it was my state of mind at the time.” Since he had had them for so long, he did not think quitting would make a difference.
“This is, of course, an inflammatory topic. There is skepticism either way,” says Lo. “But in my experience, it’s very simple. If you’re a guy and you’re worried about gynecomastia, you shouldn’t smoke pot because there’s a link.”
“That is the prevailing opinion,” says Robert X. Murphy Jr., president of the American Society of Plastic Surgeons, noting that it matches his own clinical experience. With the recent increase in men seeking breast reduction surgery, he says, empirical evidence is mounting.
In 2012, the society reports, 20,723 gynecomastia procedures were performed, a 5 percent increase from the year before. The number of these surgeries had fallen from their peak in the late 1990s, when more insurers were willing to cover the procedure. Patients now pay between $3,000 and $8,000 out of pocket for the operation. Since 2006, it has been among the top five cosmetic surgical procedures for men.
Emily Pollard, head of plastic surgery for Lankenau Medical Center, now performs one gynecomastia surgery a month, twice as many as the year before. The increase, she believes, is partly driven by direct marketing by companies that manufacture liposuction equipment.
The largest percentage of gynecomastia cases have no clearly identifiable cause, says Murphy. The rest are caused by a constellation of conditions. It is common for boys like Michael to develop tender and enlarged breasts during puberty, although, as his pediatrician said, most outgrow the condition. Additionally, more than 90 drugs have been linked to gynecomastia, including some antidepressants and antibiotics and ulcer, heart, and HIV medications. Men who are obese are susceptible. So are bodybuilders who use anabolic steroids, men who use Propecia to prevent hair loss, and those who self-administer testosterone, which the body breaks down into two compounds, one of which is similar to estrogen. Because people may be exposed to multiple risk factors, it can be difficult to identify which are to blame.
“We can’t paint with a broad stroke,” says Murphy. But when young men come to him to remove their breasts, and they are neither overweight nor taking any other of the trigger medications, he says, it is reasonable to deduce that pot is the likely cause.
“It is one of those things that you ask about,” he says. “Whether people admit it or not is another matter.”
Living with gynecomastia can be a psychological burden. “I was scared of relationships,” says a patient who gave his middle name, Andrew. “It really was a strain, every day.”
A 26-year-old drug and alcohol counselor, he says he rarely took off his shirt at the beach and would layer T-shirts to hide his body. “My friends would tease me. They thought I could make it go away with exercise.”
Unable to afford the surgery, Andrew borrowed the money for the $5,500 procedure. He had his surgery last month. “I’m thrilled with it,” he says. “It’s such a relief.”
The surgery is normally an outpatient procedure that takes about an hour to complete, says Lo. The surgeon cuts around the nipple, removes 90 to 95 percent of the glandular tissue, and contours the chest.
The remaining tissue can still be stimulated to grow, Lo says. “So we obviously advise to avoid the factors that caused it in the first place.” Marijuana, he says, is one of them.
“I’m not hating on pot,” Lo says. “Everyone who does what I do has seen it, whether you choose to believe it or not.”
Read more: http://medcitynews.com/2013/11/plastic-surgeons-link-bewteen-man-boobs-pot-real/#ixzz2re6AvPIL
The American Society of Plastic Surgeons is now collaborating with the CDC, in order to effectively disseminate this information to the ASPS membership.
“These surgical-site infections represent a serious public health problem affecting patients who opt for low-cost cosmetic plastic surgery procedures overseas, in this case, the Dominican Republic,” says ASPS Patient Safety Committee Chair C. Bob Basu, MD, MPH. “Medical tourism may attract patients with ‘cheap deals,’ but unfortunately, these deals may compromise, or worse, completely ignore recognized quality and safety standards.
No deaths have occurred.
Others who may have undergone surgical procedures in the Dominican Republic may be at risk for the “rapidly growing non-tuberculous mycobacterium” (RG-NTM) infections.
“It is possible that additional infected patients have not yet been reported,” notes Nguyen.