Minimally Invasive Facial Rejuvenation
Current Concepts and Future Expectations
Expert Rev Dermatol. 2013;8(5):565-580.
Abstract and Introduction
Abstract
Aging of the skin is a multifactorial phenomenon in which ongoing intrinsic changes combine the cumulative effects of chronic exposure to the elements, primarily UV radiation, in a synergistic fashion, causing the skin to lose its thickness and elasticity and develop wrinkles. There is now an increased interest in a wide range of non-ablative treatments for skin aging, which are used to rejuvenate skin with minimal downtime and complications. As the demand for minimally invasive rejuvenation is increasing, different modalities have been designed to produce favorable alterations in the dermis with no epidermal damage via photomodulation, selective photothermolysis, fractional photothermolysis, radio waves, electro-optical synergy, injectable fillers, neurotoxins, skin needling and biorejuvenation to stimulate collagen synthesis and rejuvenate the aged skin while preserving the integrity of the epidermis.
Introduction
Aside from being the largest organ of the human body, skin is also the only organ continually exposed to the surrounding world, interacting with the environment and reflecting the general health condition and age changes. [1]
Understanding the mechanisms by which the skin ages has been increasing significantly, along with considerable progress on the way to prevent and reverse the visible signs of aging. However, there are still several mysterious factors concerning aging process and why we all appear to age differently. [2] Aging of the skin is likely caused by both intrinsic (biologic) ‘intrinsic aging’, and extrinsic (environmental) factors ‘extrinsic or photoaging’; these factors are interconnected and may share a final common pathway. [3] The quality of skin features is greatly affected by aging, as skin ages, it tends to become roughened, lax and wrinkled with some telangiectasia and pigmentary changes. [1,4,5]
The main histological feature of photodamaged skin is solar elastosis; with accumulation of elastotic material in the papillary and middle dermis. Meanwhile, photoaged skin shows gradual decrease in collagen content. [6] Additionally, collagen network becomes disordered with decreased synthesis and enhanced breakdown. [7] These changes contribute to the skin laxity and wrinkling formation. [8]
Besides being an art, facial rejuvenation is a developing science. Patients now routinely present to their physician requesting information on improving the signs of facial aging; it is the physician’s responsibility to select the most appropriate intervention(s) based on the patient’s age, physical needs and concerns, extent and location of volume loss and cosmetic goals. [9,10] Different therapeutic approaches were used throughout the years to give the face a youthful appearance. However, because each person is unique, there is no one modality that is best for everyone. [11] Therefore, to choose the most appropriate therapy, distinctions must be done between rhytides caused by loss of collagen within the dermis, wrinkles due to volumetric loss of fat, redundant folds created by gravitational pull and those caused by hyperfunctional facial muscles. [12]
For ease of patient education, the treatment options for addressing these changes may be simplified into five categories, often referred to as the ‘5 Rs (Redraping, Resurfacing, Retaining, Relaxing and Refilling) of skin rejuvenation’: surgically Redraping and lifting redundant tissue; Resurfacing photoaged skin with ablative or non-ablative technologies whether physical, chemical or mechanical; Retaining with skin care; Relaxing dynamic rhytides that are due to hyperfunctional muscles with neurotoxins and Refilling of diminished subcutaneous tissue by restoring 3D volume. [13,14]
Although ablative modalities remain the gold principle for photodamaged skin rejuvenation, its use is associated with significant risk of side effects as well as a prolonged and an unpleasant post-treatment ‘downtime’ and recovery period. [15]Thus, interest in ablative treatment has waned considerably while non-ablative modalities as well as fractional skin rejuvenation have become appealing alternative treatments. [16]
New perspectives in non-ablative skin rejuvenation treatments have been established with the development of new technologies and techniques, which are used to rejuvenate skin with minimal downtime and complications. [3,17] Many different terms have been used to describe these procedures including: subsurface resurfacing, laser toning and minimally invasive skin rejuvenation. These modalities are designed to produce many cosmetic benefits, including improvement of wrinkles, skin laxity and texture. [18]
Beside lasers and various in-office procedures, many topical skin care agents were used for prophylaxis as sun screens and for rejuvenation such as retinoic acid and different anti-oxidants including vitamins C and E, co-enzyme Q10 and green tea.[19]
Minimally Invasive Modalities for Skin Rejuvenation
Choosing the appropriate treatment modality which will be the key to success in skin rejuvenation depends on careful evaluation and determining the patient’s needs, skin type and condition, to frame a treatment plan. [20] Good candidates for minimally invasive techniques tend to have minimal facial sagging. Patients should understand that skin texture will improve and fine lines will be softened but not eradicated. Cumulative aesthetic benefits will occur gradually and will be less dramatic than those seen with ablative resurfacing. [18] Patients with Fitzpatrick skin type III or less are generally best candidates for different procedures with minimal risk of complications. [21,22]
The goal of most minimally invasive treatments is to induce selective dermal injury which results in wound repair response; while keeping the overlying epidermis intact. [18] In response to the induced dermal injury, the healing process begins to stimulate the fibroblast with deposition and reorientation of collagen bundles. [23] Such modalities for skin rejuvenation could be classified into two types, the first relates to treatment of ectatic vessels, pigmentation and pilosebaceous changes, while the second refers to dermal remodeling with wrinkle reduction and/or skin tightening. [24,25]
Minimally invasive skin rejuvenation techniques could be categorized into several different general modalities including: non-ablative laser technologies and light sources, non-laser modalities (radiofrequency [RF] systems and ultrasounds), electro-optical synergy (ELOS) technique beside other approaches and procedures (superficial chemical peels, microdermabrasion, injectable fillers, neurotoxins, skin needling, mesotherapy, platelet-rich plasma [PRP] and stem cell therapy) (Figure 1).
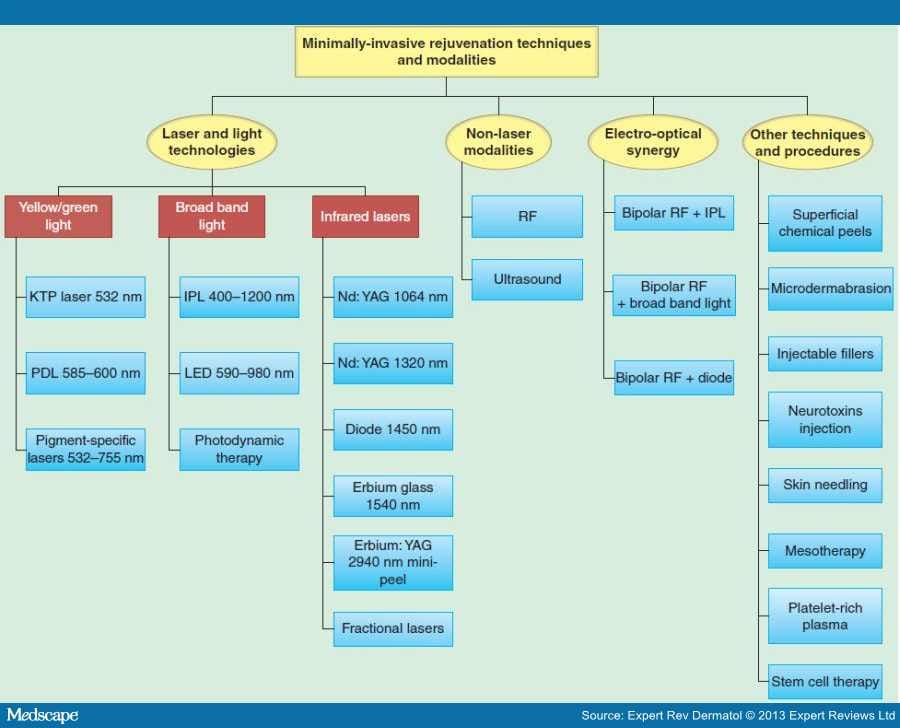
Figure 1.
Minimally invasive techniques and modalities for skin rejuvenation.
IPL: Intense pulsed light; KTP: Potassium titanyl phosphate; LED: Light-emitting diode; PDL: Pulsed dye lasers; RF: Radiofrequency.
Non-ablative Laser Technologies & Light Sources
Lasers and light sources used for non-ablative photorejuvenation could be classified based on their wavelengths into: i) yellow to green light, ii) systems emitting broad band light and iii) those emitting light in the infrared range (infrared lasers target pigment, hemoglobin and water).
Potassium Titanyl Phosphate Laser 532 nm. The potassium titanyl phosphate (KTP) laser uses a 1064 nm neodymium:yttrium-aluminum garnet (Nd:YAG) source passed through a KTP crystal to emit light with a wavelength of 532 nm. [26] This green light wavelength is absorbed by both hemoglobin and melanin. As a result, both unwanted vessels and pigment can be treated. At the same time, textural improvement is also seen, but to a much lesser extent. [18]
The KTP laser’s 532 nm wavelength corresponds with the 542 nm absorption peak of hemoglobin, which makes it relatively specific for cutaneous blood vessels. [26] Single vessels are traced by using a spot size close to the same vessel’s diameter. This will bring energy only to the targeted vessel and closely nearby tissues, leading to photocoagulation without extravasation of the vessel content, with subsequent sparing of normal capillaries. [27,28]
On comparing green with yellow light, the high absorption of 532 nm KTP by melanin is the only theoretical disadvantage, making it unsuitable for treatment of darker skin types. [29,30]
Pulsed Dye Laser. Pulsed dye lasers (PDL) emit yellow light at 585–595 nm which selectively targets hemoglobin and melanin. This wavelength permits a 50% dermal penetration with 400 μm depth, and is exclusively absorbed by blood vessels. Thus, enhancing the release of inflammatory mediators from endothelial cells within the targeted vessel with subsequent stimulation of fibroblast activity to produce new collagen. [24,31]
Many studies suggested the potential role of PDLs in the treatment of photodamaged skin by the clear clinical and histologic improvement seen with PDL-treated patients. [15] Improvement in the appearance of wrinkles has been observed following exposure to short pulsed 585 nm laser light at low energy levels (2–3 J/cm 2). [25] Longer pulses theoretically allow more heating of larger capillaries with less risk of purpura, thus reducing the downtime. [32]
Despite approval by the US FDA for treating photodamage with the PDL, only modest results have been observed with these wavelengths, presumably because of predominantly vascular targeting and superficial penetration to the papillary dermis. [15]
Pigment-specific Lasers. Pigment-specific lasers are used to treat the pigmentary changes that occur with photodamage, including solar lentigines, ephelides or freckles. These include Q-switched (QS) Nd:YAG (532 nm), QS ruby (694 nm) and QS alexandrite (755 nm) lasers as well as QS 1064 nm (infrared spectrum) laser. [8,24]
The frequency doubled Nd:YAG laser emits radiation with a wavelength at 532 nm and a pulse duration in nanoseconds. The use of QS lasers in the treatment of pigmented lesions follows the principle of selective photothermolysis thus limiting the damage to the melanosome-containing cells. However, at 532 nm, the wavelength is absorbed not only by melanin but also by hemoglobin. [24,33]
Ruby laser 694 nm with 28–50 ns pulse duration, was the first QS laser system produced for epidermal and dermal pigmented lesions; however, caution is a must as patients with darker skin types can develop permanent hypopigmentation. [33,34]
The QS alexandrite 755 nm laser with 50–100 ns (slightly longer pulse duration) is used to treat lentigines with less epidermal disruption. Whitening without ablation of the epidermis is usually the treatment end point. [35] Bruising, crusting as well as temporary and permanent pigmentary changes are not uncommon side effects, leading to some longer downtime. [33,36]
Intense Pulsed Light. As one of light-based technologies, intense pulsed light (IPL) is used to rejuvenate aging skin; it emits a non-coherent polychromatic light with filtered flashlamp source in a broadband wavelength (400–1200 nm) in the visible and mid-infrared ranges of the electromagnetic spectrum. Meanwhile, cutoff filters are used to allow a defined wavelength to penetrate the skin and target particular structures. [37,38] The device is capable of emitting yellow, red and infrared simultaneously so that multiple components of photoaging can be treated concurrently. [39,40]
Similar to lasers, IPL systems produce their effect based on the principle of selective photothermolysis. Unlike lasers, which treat one chromophore with monochromatic light, IPL systems have wide spectrum of probable combinations of wavelengths, pulse durations, pulse intervals and fluencies. IPLs have the ability to target both melanin and hemoglobin; thus treating vascular and pigmented lesions very efficiently with modest clinical improvement in wrinkles. [15,25]
IPL devices have the following advantages as they are safe effective treatments for redness or flushing of the face, neck and chest and they exert substantial visible improvement with no downtime, bruising or crusting. Disadvantages of IPL devices include their large spot sizes and bulky handpieces, making its application to small concave areas of the face difficult. Another disadvantage is the lack of real-time visibility of the treatment area due to the need of contact cooling for epidermal protection. [37,41]
Light Emitting Diode. Light-emitting diodes (LEDs) emit a narrow band of electromagnetic radiation, measured in milliwatts, ranging from the UV to the visible and infrared wavelengths. They can be classified as emitting wavelengths between lasers and broadband light. [42] An array of LED with dominant wavelength of 590–980 nm is used for treatment of photoaged skin. More specifically, they produce pulses of low energy, non-laser and non-thermal light that modulate the biologic activity of keratinocytes and fibroblasts by affecting the mitochondria, increasing collagen production. [18]
LEDs are typically assembled on small chips or equipped with tiny lenses put together into small lamps, LED is safe for all skin types and is fast and convenient to use. [31,39,42] Although the biological effects on skin cells seem to be evident (wound healing, reduction of chronic and acute actinic damage), clinical results of skin rejuvenation obtained simply with LED photomodulation are not particularly convincing either on skin tonus or on wrinkle reduction. [33,43]
Photodynamic Therapy. Photodynamic therapy (PDT) is defined as a photochemical reaction used to selectively destroy tissue. It is considered as a form of photochemotherapy that uses a photosensitizer, light and oxygen. [44] The use of PDT for skin rejuvenation has been well documented in different studies. It is a two-stage therapeutic technique in which the use of a topical (5-aminolevulinic acid (5-ALA; 20%) or methyl aminolevulinate [MAL]) or systemic (hematoporphyrin [Hp], hematoporphyrin derivative [HpD] or systemic 5-ALA) sensitizing drug, is followed by visible light radiation of appropriate wavelength (IPL, LEDs, PDL and blue light [410–490 nm]). [45]
The photosensitizers, administered exogenously or formed endogenously, are activated by the light and transfer energy to molecular oxygen, thereby generating reactive oxygen species to induce cell death. [46,47] Protoporphyrin IX has its largest absorption peak in the blue region at 410 nm with smaller absorption peaks at 505, 540, 580 and 630 nm. However, a blue fluorescent lamp (peak emission 417 nm) is used in Levulan-PDT. [48,49] The majority of clinical studies are performed using light wavelengths of 625–633 nm, which permit greater skin penetration. The effective therapeutic depth appears to be close to 1–3 mm when 635 nm is used. This is due to the capacity to produce a photodynamic reaction, which also depends on the dose of light and also on the quantity of photosensitizer used in the target tissue. [45,50]
Different studies suggest that PDT may improve the appearance of wrinkles and fine lines, telangiectasias, skin tone and photodamage. For the use of IPL in PDT, usually handpieces with a cut-off filter allowing transmission of light above 600 nm (used for hair removal) are suitable. Pulse duration can be set at a large range (millisecond). Short pulse duration plays a role particularly with respect to pain. [51] In comparison with continual irradiation with red light, PDT with a flash lamp is perceived as less painful. The various probable parameters of IPL with respect to wavelength, pulse duration, pulse interval and energy density make targeted use possible for the experienced dermatologist, on one hand, but make the comparison of different studies difficult on the other. [52,53]
Nd:YAG Laser, 1064 nm Long Pulsed & Short Pulsed Q-switched. The long pulsed Nd:YAG emits energy in infrared spectrum at 1064 nm with extended pulse duration. The laser results in diffuse heating of dermal tissue caused by the deeply penetrating nature of 1064 nm, which has an optical penetration depth of 5–10 mm. [39] The chromophores for the 1064 nm laser are, in decreasing order, melanin, hemoglobin and water. Water weakly absorbs laser energy at this wavelength and is gently heated; however, severe heating remains localized to hemoglobin and melanin. [24,43]
QS 1064 nm Nd:YAG is one of the first lasers used for non-ablative skin rejuvenation. Its long wavelength and ultra-short pulse duration of nanosecond, allow penetration of the papillary dermis with subsequent dermal wounding and limited thermal diffusion to the proposed target. Multiple treatments are required to attain best results. [30,54]
Facial telangiectasia (spider veins) and mild photodamaged skin are main clinical indications. [43,55] The QS 1064 nm Nd:YAG laser could be also used for treating pigmentary changes beside vascular changes that occur with photodamage, because it highly targets melanin within dermal melanocytes. [24,33] Meanwhile, side effects include mild erythema in all patients (lasting from 1 to 2 h), purpura, rarely post-inflammatory hyperpigmentation and temporary hypopigmentation, can be avoided by using lower fluencies. [31,55]
Nd:YAG 1320 nm Laser. The 1320 nm laser system was the first available system designed exclusively for selective dermal heating. The primary chromophore of the 1320 wavelength is dermal water; which is well scattered horizontally and vertically, thus allowing for maximal dermal injury. [18,22,31]
The Nd:YAG 1320 nm laser has beneficial effect in reversing the signs of skin aging at both the clinical and histological levels. [54,56–58] At the clinical level, Nd:YAG enhances skin tightening and reduces wrinkles. At the histological level, Nd:YAG enhances the formation of newly synthesized collagen and increases the dermal matrix contents with improving the morphologic appearance of collagen I and III together with elastic fibers. [58]
Diode Laser 1450 nm. The 1450 nm diode laser produces low peak powers (10–15 W); that means relatively long exposures are necessary in order to achieve sufficient fluences for selective dermal heating. These longer pulse durations require cooling to be delivered in a sequence of sprays before, during and after the pulse. [59]
In the infrared portion of electromagnetic spectrum (>700 nm), the water absorption coefficient is relatively low, [60] allowing this infrared technology to target tissue water and penetrates skin to a depth of approximately 500 μm. It is used for facial rejuvenation targeting the water in the upper dermis, this laser remodels the skin’s underlying collagen and promotes formation of new collagen, improving facial and periorbital rhytides. Patient acceptance of the treatment was high, but most felt that there was little improvement of the treated rhytides. [18,39] Side effects are usually minimal and can include postoperative erythema, edema and hyperpigmentation in patients with darker skin type. [24,60]
Erbium: Glass Laser 1540 nm. The erbium:glass 1540 nm laser is a flash lamp-pumped system with yttrium-erbium phosphate glass. Similar to other infrared lasers, its wavelength is highly absorbed by water but minimally by melanin. The wavelength is delivered in 10–100 ms pulses with fluences ranging from 20 to 30 J/cm 2. [39,61] The skin is cooled using a handpiece; which comes in direct contact with the skin, with purified tetrafluoroethane cryogen circulating inside. The handpiece has a real-time temperature monitor at the sapphire for immediate feedback. [62]
The primary depth is within the papillary dermis where collagen tightening and neocollagenesis are achieved. The erbium:glass 1540 nm laser is used to treat a variety of conditions through the destruction of the sebaceous glands by warming up of the tissue and reducing sebum production. It is also used in skin rejuvenation, scars and acne scars by stimulating the formation of new collagen. This leads to an improvement of the skin structure as well as a reduction of wrinkles and pore size. [31,63]
Advantages of therapy include a lack of pain, discomfort or downtime. Disadvantage of therapy is that patients may have great expectations and may be disappointed with the results. Improvement is slow (occurring in months) and mild, with most patients appreciating more elastic and firmer skin. [31]
Er:YAG 2940 nm Mini-peel. Although it is utilized primarily for ablative resurfacing, erbium:yttrium-aluminum garnet (Er:YAG) laser has been used for non-ablative rejuvenation. [24,64,65] The Er:YAG laser is characterized by high absorption coefficient of its mid-infrared radiation in water; thus inducing minimal thermal injury to the underlying tissue. [66] The zone of residual thermal damage (RTD) is typically 20 ± 50 μm deep; which results in faster skin re-epithelialization. [67] This started the idea to produce deep collagen denaturation by stacking of repetitive Er:YAG pulses. [68–71]
Micro-resurfacing is a technique that employs the use of Er:YAG laser system to deliver a single-pass ‘mini-peel’. The use of a sequence of short Er:YAG pulses (200–270 ms) below the ablation threshold increases the temperature in the upper dermis to about 60°C in order to induce collagen denaturation. [72,73] Benefits of this technique include that it is an effective, well-tolerated and minimally invasive treatment option for photoaging as it stimulates collagen formation and remodeling of extracellular matrix (ECM) proteins without ablation of the epidermis. This is accompanied by a noticeable clinical improvement of wrinkles and photoaged skin with the advantage of minimal downtime and side effects. [64,65]
Multiple passes over the wrinkles result in a thermal build up by heat conduction. As a result, the optical penetration depth is increased; resulting in further diminished ablation efficiency, enhanced deposition of heat and increased zone of thermal injury. [64,74,75]
A recent study by El-Domyati et al. showed the effect of multiple passes using Er:YAG 2940 nm laser mini-peel on subjects who were treated on the face every 2 weeks for 3 months for a total of six sessions. [65] A moderate clinical improvement (Figure 2), accompanied with significant histologic findings in the form of increased types I and III collagen and decreased dermal elastin in response to treatment was reported.
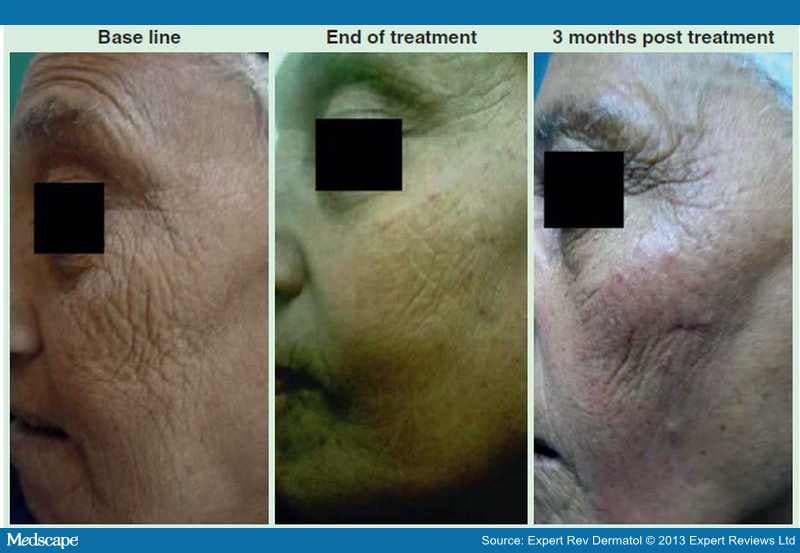
Figure 2.
Representative photographs of a patient treated with Er:YAG 2940 nm laser mini-peel showing moderate improvement of wrinkles in response to treatment.
Fractional Lasers. Fractional photothermolysis is a novel technology for skin rejuvenation that can be considered intermediary between ablative and non-ablative resurfacing; it could be achieved with non-ablative and ablative modalities. [76]
True non-ablative fractional laser requires three criteria: i) non-ablative mode of tissue coagulation, with preserving the stratum corneum, ii) creation of multiple microthermal zones (MTZs) surrounded by islands of viable tissue and iii) resurfacing with extrusion and replacement of damaged tissue, with re-epithelialization within 24 h. [77,78] With this technology, fractional lasers are employed creating thousands of tiny treatment zones on the skin, microscopic columns of thermal injury (microthermal zones), the depth of penetration ranges from 300 to 700 μm based on fluencies. [77,79,80] The target chromophore for the fractional laser is water; however, treatment is performed in a pixilated fashion, leaving approximately 70% of the skin undamaged to promote rapid healing. The wound-healing response differs from that of other techniques because viable cells exist between treatment zones, including epidermal stem cells and transient amplifying cell populations.[31] Each laser hit produces a 30–70 μm plug of microscopic epidermal necrotic debris that naturally exfoliates in approximately 14 days. [81]
Relative epidermal and follicular structure sparing is responsible for rapid recovery without prolonged downtime. Melanin is not at risk of selective targeted destruction; therefore, fractional resurfacing has been used successfully in patients with dark skin color. [57,76] Dermal effects of microthermal zone repair generate wound mediators that ultimately lead to remodeling of the dermal matrix and histologic demonstration of enhanced rete ridge which enhance skin rejuvenation. [76,82]
Ablative fractional modalities are laser systems using an ablative laser (Er:YAG or carbon dioxide) pulse that only hits a fraction of the skin at each pulse. This allows many skip areas in between the MTZs to quickly re-epithelialize the wounded skin. MTZs allow delivery of high local irradiance to achieve efficacy while maintaining low overall irradiance to prevent side effects. [82] Unlike non-ablative fractional photothermolysis, these devices cause true ablation of the epidermis in addition to variable depths of ablative damage to the dermis. The combination of epidermal and dermal ablation appears to lead to a more robust wound-healing response and accompanying dermal fibrosis, which may explain the rapid and significant clinical effects that can be achieved with ablative versus non-ablative devices. [83]
Complications with non-ablative fractional laser resurfacing are rare and generally self-limiting. Prolonged erythema has been reported with higher fluencies, but generally resolves. Microthermal zone pattern persistence can occur and usually resolves within 2–3 weeks. [84]
Non-laser Modalities
Radiofrequency. RF energy has been used for more than a century in a variety of medical applications. Non-ablative RF (monopolar, bipolar, tripolar or multipolar and fractional) was described by different studies as an effective and safe approach for skin rejuvenation. [3,85] Depending on the delivery system and frequency, superficial or deep heating can be produced.[18,86] RF was approved by the FDA in 2002 for the non-ablative treatment of wrinkles and skin tightening and for full-face treatment in 2004. [87,88]
Essentially, RF devices consist of a RF generator, automatic resistance test technology via computerized software, continuous cooling system, hand piece and different size treatment tips. [39,78] The RF generator produces alternating current that creates an electric field through the skin. The electric field shifts the polarity millions of times per second, causing a change in the orientation of charged particles within the electric field. Thus, heat is generated by the skin’s resistance to the flow of current within an electric field. [57,89,90]
The mechanism of action of RF is dual in nature: i) an early instant collagen contraction and ii) a secondary wound-healing response, in the form of new collagen formation and remodeling with eventually tightening. The immediate skin contraction is attributed to the sparse pattern of collagen denaturation, while sufficient tissue is left healthy to ensure wound healing. [3,91]Over time, as a thermally mediated healing response, heated fibroblast stimulation enhances formation of new collagen leading to further collagen tightening and overall increase in collagen deposition. [92] Additionally, another mechanism of action for monopolar RF has been based on the fact that the delivered energy usually favors the least resistance path. As subcutaneous fat lobules are divided by interweaving network of collagen-based septa, these fibrous septa are preferentially heated resulting in collagen tightening. [93,94] This gives the patient immediate, visible improvement the day of the procedure with subsequent lifting and remodeling of subcutaneous tissue, as well as the skin becomes tightly attached to the underlying structures. [94,95]
Unlike most lasers, which target specific chromophores, the output energy of the RF is a chromophore-independent; it does not follow the principles of selective photothermolysis. Heat is generated as a result of tissue resistance to the movement of electrons within the RF field; [90] allowing energy to be delivered to 3D levels of the dermis. [16,90]
El-Domyati et al. analyzed the effect of monopolar RF on individuals who underwent treatment on the face every 2 weeks for 3 months for a total of six sessions. [3] Punch biopsies of the facial skin were performed at baseline, end of treatment and 3 months after treatment. Noticeable clinical improvement (Figure 3), together with significant histologic findings including decreased dermal elastin as well as increased collagen types I and III was observed after treatment.
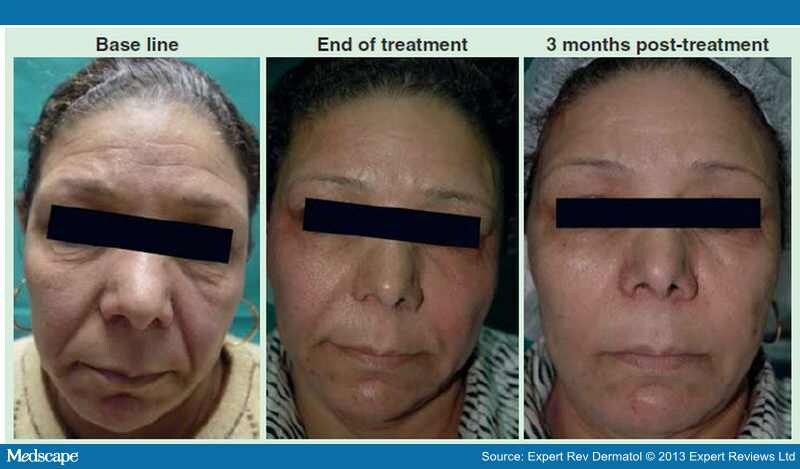
Figure 3.
Clinical response to monopolar radiofrequency treatment showing improvement of forehead, periorbital and nasolabial areas at end of treatment with continued improvement at 3 months post-treatment, compared with base line.
Bipolar RF devices pass electrical current only between two positioned electrodes applied to the skin. No grounding pad is necessary with these systems, as monopolar RF, because no current flows throughout the remainder of the body. It is claimed, however, that bipolar RF cannot produce a uniform volumetric heating comparable at all with monopolar RF. [37,91,96]
The use of a tripolar (multipolar) device has been explored for the treatment of skin aging; the device has three probes that exploit the benefits of both unipolar and bipolar RF to treat tissue. Although bipolar devices rely on active integrated cooling devices to avoid damage to the epidermis, the tripolar device advertises simultaneous moderate deep and superficial heating of tissues that does not require protective cooling for the epidermis. [97]
Newer RF technologies including devices with multigenerational sources and fractional RF system have emerged with more promising potential since then. Fractional RF has the non-invasive modes of known RF devices with the development of a minimally invasive bipolar microneedle delivery system. It generates localized coagulation zones within the reticular dermis characterized as RF thermal zones. This fractional RF system offers controlled dermal heating through pulse duration variance, allowing for fractional sparing of the epidermis and important adnexal structures. [78,96] Studies showed that the treatment-generated RF thermal zone in the reticular dermis consists of denatured collagen separated by spared dermis. These zones were replaced by new dermal tissue within 10 weeks. [96]
Ultrasound. Ultrasound devices were first approved for eyebrow lifting in the USA in 2009, and have subsequently been used for treatment of skin and tissue laxity. [98] This modality uses ultrasonic energy to produce specific micro-coagulation zones deep in the dermis and subcutaneous adipose tissue. During the months following treatment, repair of the deep tissue damage leads to contraction and tissue remodeling, resulting in the desired aesthetic effect of reduced skin laxity. [99,100] The superficial dermis and collateral tissues are spared, which not only decreases the risk of scarring and downtime but potentially permits to be used in different skin types. [98–101]
Electro-optical Synergy
ELOS, combined electrical and optical energy, is a new technology which has been recently introduced as a non-ablative treatment for skin rejuvenation. This technology uses RF and optical energy from laser or light sources within the same device to be combined in the same pulse profile. It is based on the principle of synergistic activity between the two forms of energy. [10,90,102]
The ELOS system consists of a bipolar RF generator and a flashlamp. The pulsed light is delivered through a contact sapphire light guide with the bipolar RF energy which is delivered through electrodes embedded in the system applicator and brought into contact with the skin surface. The temperature of the tissue is continuously measured throughout the duration of the pulse to prevent overheating and improve safety. Some devices use non-laser light source (580–980 nm) for optical energy, whereas others use a high-power diode laser (900 nm) as its light source. [102–104]
The mechanism of this new technology is based on two combined approaches: the first approach includes light-based technologies where the pulsed optical energy targets and preheats distinct chromophores producing differences in temperature between the target and the surrounding tissue, according to the principle of selective photothermolysis. [10,105]The second approach uses the creation of stress waves at the skin surface by RF inducing uniform heat at controlled depth to dermal layers. The RF energy usually follows the least resistance path, a phenomenon known as impedance. [10,16]
The generated heat by pulsed optical energy will reduce the target impedance providing favored conduction pathway for the RF toward the target of interest. [106] As a result, the combined forms of energy act synergistically to initiate thermal wound at the targeted area with subsequent remodeling and reorientation of collagen bundles and formation of new collagen; which are achieved over months after treatment. [90,94,107]
A recent study by El-Domyati et al. evaluated the histologic changes and corresponding clinical outcomes after use of the ELOS technique (RF combined with IPL) for skin rejuvenation. [10] The volunteers were treated for a total of six sessions at 2-week intervals. The outcomes were assessed using photographs and punch biopsies taken at the end of the treatment and 3 months post-treatment, that showed improvements in skin tightening, texture and wrinkles, which was accompanied by reduction in elastin 3 months after treatment and a significant increase in collagen types I and III (Figure 4).
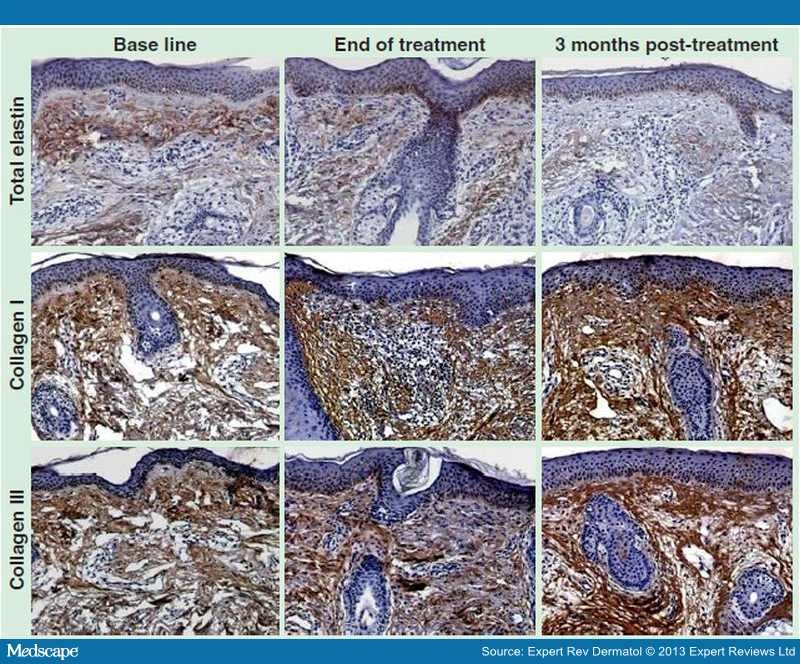
Figure 4.
Dermal elastin and collagen contents in response to electro-optical synergy treatment. Immunoperoxidase staining of skin tissues at base line (left panels), end of treatment (middle panels) and 3 months post-electro-optical synergy treatment (ELOS) treatment (right panels), for total elastin (top row) and collagen types I and III (middle and bottom rows); showing a decrease in dermal elastin upon ELOS treatment (top row) with an increase in collagen type I (middle row) and type III (bottom row) contents in response to ELOS (immunoperoxidase, x200 magnification).
The bipolar RF component enables the use of lower levels of the optical component, reducing the risk of optical energy and potentially improving its use across different skin types. The optical component is believed to drive the bipolar RF energy to concentrate where the optical energy has selectively heated the target. [102,108] This technology appears to be an ideal option for darker skin types due to the fact that optical energy has weak absorption of melanin and RF energy does not depend on chromophores for its effects. [22] It can be used to rejuvenate the aged skin and reverse the signs of photoaging safely and effectively. This modality stimulates the remodeling process, and improves the clinical and histological signs of aging, with the advantage of being a safe procedure and avoiding significant downtime. [10]
Side effects associated with combined electro-optical energy are uncommon. [104,109] The use of lower energy levels allows patients to tolerate the procedure well with minimal discomfort and no need for adjunctive anesthesia. The most common side effect is transient erythema immediately after the procedure, usually resolving in minutes to hours. Crusting, blisters, pigmentary change and scarring are rare. [90,108]
Other Minimally Invasive Techniques & Procedures
Chemical Peeling. Chemical peeling is the topical application of chemical agents to the skin, which causes controlled destruction of part or the entire epidermis, with or without the dermis, leading to exfoliation and removal of superficial lesions, followed by regeneration of new epidermal and dermal tissues. [7]
Chemical peels can be classified into: i) very superficial (exfoliation) which includes destruction of the stratum corneum without creating a wound below the stratum granulosum, ii) superficial (epidermal) including destruction of part or all of the epidermis, anywhere from the stratum granulosum to the basal cell layer, iii) medium (papillary dermal) with destruction of the epidermis and part or all of the papillary dermis and iv) deep (reticular dermal) which includes removal of the epidermis and papillary dermis, extending into the reticular dermis. [110,111]
Superficial chemical peels are currently performed with various compounds including trichloroacetic acid (TCA), alpha hydroxy acid (glycolic acid) and Jessner’s solution (14% lactic acid, 14% resorcinol and 14% salicylic acid in ethanol). [17] TCA produces superficial peeling (minimally invasive) when used in dilutions from 10 to 35%. [112,113]
Indications for chemical peeling include pigmentary disorders, acne and superficial acne scars, skin aging and benign epidermal growths. Relative contraindications may include dark skin type of the patient and patient with unrealistic expectations. [114] Meanwhile, absolute contraindications include active bacterial, viral or fungal infection, open wounds, pre-existing inflammatory dermatoses, non-cooperative patient (regarding sun exposure or application of sun block) and history of abnormal scarring, keloids, atrophic skin or isotretinoin use in the last 6 months. [86]
Microdermabrasion. The concept of facial resurfacing was reported as early as 1500 BC, when the Egyptians used sandpaper to smooth scars. Dermabrasion, the technique that penetrates to the depth of the dermis to promote skin regrowth and rejuvenation, was developed in the early 1900s and modified to its modern form in the 1950s. [115,116]
Microdermabrasion or particle resurfacing was designed in the 1980s and gained popularity because of its ease of use, relatively benign nature and its proposed gross effectiveness. It is a minimally invasive procedure that relies on an abrasive component and a vacuum tube causing mechanical removal of the superficial epidermis and stimulation of new cell growth.[117]
Different microdermabrasion systems are known based on the source of the abrasive component. Most systems use inert crystals usually aluminum oxide, sodium chloride or sodium bicarbonate crystals that is propelled to the skin surface using a handpiece. In other systems, the coarse stimulus is a handpiece with rough crystals (diamonds); these crystals are fixed to the surface of handpiece which comes in direct contact with the skin. [118,119]
Vacuum suction is concurrently used to gather worn-out crystals and debris which are caused by the crystals’ abrasive action and transferred via a separate set of collection tubing into a dissipate container. [120,121]
Several authors have illustrated that microdermabrasion may play a role in the improvement of skin contour irregularities, including rhytides via mechanical removal of the stratum corneum and stimulation of new cell growth. Although microdermabrasion can be used to improve certain pigmentary changes, it might be less effective when compared with chemical peels. [111,122,123]
The side effects associated with microdermabrasion are minimal, the majority of patients may develop erythema and mild pain, meanwhile minor abrasions and petechiae may occur if the procedure is carried out aggressively. Yet, they are usually short-lived, lasting about 1 week. [117,119] Microdermabrasion is considered to be safe in all skin types, and the risk of post-inflammatory pigmentary changes is minimal. [111]
Injectable Fillers. Dermal fillers are one of the most common and useful treatments for wrinkles and folds. Over the past few years, there have been tremendous advances in the use of fillers. They have provided enhanced results as well as longevity for treating folds, wrinkles, pitted scars and skin depressions of the face bringing back its fullness. [124,125]
Fillers can be classified according to their permanence in tissues into: permanent, semipermanent and temporary. The majority of injectable fillers are temporary, lasting from several weeks (short lived) to several months (long acting); which require ongoing treatment to maintain the desired appearance. [126,127] Permanent fillers remain in the site of inoculation for years, but they are no longer suggested to be used, as their long-lasting safety has not been established; on the other hand, some other studies have revealed outstanding results for the use of these permanent fillers. [128]
Temporary fillers contain different substances including collagen and hyaluronic acid (HA) which is the most commonly used material. HA polysaccharide is a natural component of human dermis and epidermis, consequently, HA-based fillers have exceptional biocompatibility while providing indistinguishable structural as well as mechanical properties of normal human subcutaneous tissue. Hyaluronan, a naturally occurring substance, is rapidly broken down by hyaluronidase enzyme and metabolized to carbon dioxide and water after a short half-life of about 12 h. Thus, cross-linking increases HA fillers ( in vivo) lifespan from 6 to 18 months. [126] Factors that impact HA persistence include HA concentration, percentage and type of cross-linkage, water binding capacity and injection technique. [128]
Some filler substances such as polylactic acid or hydroxylapatite have long half-life. Fillers composed of polyacrylamide hydrogel and polyalkylamide remain permanently in the soft tissues, as well as liquid silicon. Although the subcutaneous injection of liquid silicone was declared illegal in many countries, it is still in use because of the low cost compared with other fillers. [129]
Minimally invasive dermal fillers used alone or in combination with botulinum toxin (BTX), have accounted for much of the surge in non-surgical facial rejuvenation procedures. [124] For motion wrinkles (dynamic lines caused by facial movements), the optimal tactics in many cases is to try BTX first. In fact, even if BTX has failed, it may still be a good adjunct to filler because simply injecting filler does not eliminate the cause of a motion wrinkle. [129–132]
Although fillers are generally regarded as safe, adverse outcomes can occur with these agents. Superficial placement of dermal fillers is a common error and is associated with a range of complications, from obviously visible product to inflammatory nodule formation and even hypertrophic scarring. Furthermore, complications may include unrealistic patient expectations, bruising/hematoma, undercorrection, overcorrection, asymmetry, lumping, granuloma and iatrogenic as well as allergic reaction. [124]
Botulinum Toxin. BTX is an injectable medication that relaxes the overactive contracting muscles to treat wrinkles mainly in the upper face. It was discovered as a toxin created by the Clostridium botulinum bacterium that causes food poisoning and muscle weakness; this toxin inhibits the release of acetylcholine from neuromuscular presynaptic vesicles producing a temporary muscle paralysis. [133,134] Thus, the muscles cannot contract to enhance existing wrinkles or to encourage new ones. This results in a much smoother, younger-looking appearance for the face. [134,135]
There are seven distinct serotypes of BTX: A, B, C1, D, E, F and G. Serotypes A and B (BTX-A and BTX-B) are available commercially. Botulinum toxin A (BTX-A) has successfully been used in the treatment of hyperkinetic facial rhytides since the late 1980s, while BTX-B is less commonly used and data regarding the efficacy and optimum doses are few. [110]
Tiny amounts of BTX are injected directly into the muscle that is causing wrinkles or dynamic lines. These injections are adjusted in doses of International Units (IU). [135,136] BTX injections are safe when done properly by an experienced and licensed physician who is an expert in facial anti-aging therapy and aware of muscle anatomy. However, there are potential side effects which can occur, some are unavoidable and others are related to injection technique. The most common potential sequelae of BTX are: overcorrection, undercorrection, asymmetric result, upper eyelid ptosis, neck weakness, perioral droop, bruising, intravascular injection, diplopia (lateral rectus) and psychosomatic problems. [130,133,137]
Microneedling Therapy. Microneedling therapy, also known as percutaneous collagen induction (PCI) therapy, is a recent addition to the treatment armamentarium for skin rejuvenation. The treatment is performed as an office procedure after application of a local anesthetic cream, by means of an instrument known as a dermaroller. [138]
A dermaroller is a simple, hand-held instrument consisting of a handle with a cylinder studded all around with fine, stainless steel needles 0.5–2 mm in length. This needle-studded cylinder is rolled on the skin in multiple directions to achieve a therapeutic benefit and hence the name ‘dermaroller’. [139]
During treatment, the needles pierce the stratum corneum and create microconduits (holes) without damaging the epidermis. It has been shown that rolling with a dermaroller (192 needles, 200 μm length and 70 μm diameter) over an area for 15-times will result in approximately 250 holes/cm 2. Microneedling leads to the release of growth factors which stimulate the formation of new collagen (natural collagen) and elastin in the papillary dermis. [115,116]
Treatment with dermaroller is performed at 4- to 8-week intervals and multiple sessions are needed to achieve the desired effect on the skin. Microneedling or dermaroller treatment is becoming popular all over the world; the treatment can be performed in an office setting and does not need any extensive special training or expensive instruments. [139–141]
Although dermarollers are easy to use, yet there are some disadvantages such as inability to treat small areas or localized scars as the roller damages adjacent skin too. In addition, the pressure used for rolling insults the epidermis. All these difficulties led to introduction of dermastamps as well as automated microneedling devices to the market. It is a pen-like instrument with handle, disposable needles and guides (to adjust needle length). The needle tip is having 9–12 needles arranged in rows. It has different modes of speed and controlled depth. Automated microneedling devices are easy to use as the user has to just put the device in stamping action on skin and the perpendicular movement of needle leads to penetration at required depth. [142,143]
Biorejuvenation (Mesotherapy). Biorejuvenation, also called biorevitalization, is a common term of mesotherapy for skin rejuvenation. Mesotherapy is a non-surgical cosmetic medical treatment belonging to homeopathic medicine which is usually administered by alternative medical practitioners. It does not indicate a treatment of any condition in particular; it simply describes a method of drug delivery. [144,145] Mesotherapy, originally used in Europe, based from Greek word ‘mesos’ which means middle, and ‘therapeia’ that means to treat medically, has been one of the newest techniques in cosmetic medicine to rejuvenate the aging skin, and it is performed by medical and non-medical professionals. [146,147]
Mesotherapy is a minimally invasive technique that consists of intradermal injection of variable mixtures of natural plant extracts, homeopathic agents, pharmaceuticals, vitamins and other bioactive substances in microscopic quantities through multiple dermal punctures. [148] It has been used to rejuvenate (mesoglow) and eventually tone (mesolift) the injected areas of the face and other body areas like neck, low neckline (decoltage), dorsum of hands, belly and inner surface of arms and legs. The most common and simplest formulation of mesotherapy for facial skin rejuvenation involves injection of a multivitamin solution into the dermis over the course of multiple sessions. [146,149,150]
It has been claimed that intradermal vitamin injections rejuvenate the cells, making them more active and thereby stimulate the production of collagen and elastin through increasing the biosynthetic capacity of fibroblasts. Injection of hyaluronic acid is supposed to promote skin rejuvenation by increasing hydration and recreating a favorable environment to facilitate fibroblast activation and interactions between cells and ECM. [144]
There are no published clinical data on standardized reagents, treatment protocols (including dose/injection, technique/injection and interval times) or appropriate positive and negative controls and end points. Although the US FDA has approved most of the mesotherapy ingredients used in injection, the components are being applied for unapproved indications. [146,149,151] Usually, treatments are given initially as once per week for 4 weeks, once every 2 weeks for 2 months and then once per month to achieve best results. [148,152]
The performed injection techniques can be one of the following: i) intra-epidermal technique involves placing small quantities of the medicine within the epidermis. It is simple, painless and there is no bleeding, ii) papular technique involves injecting the medicine at the dermoepidermal junction, iii) Nappage technique, in this approach injections are given at a depth of 2–4 mm at an angle of 30–60° and iv) point-by-point; this is a precise single injection into the deep dermis. [147,151,152]
The controversy surrounding mesotherapy efficacy and potential adverse effects are still of concern to many researchers.[152] As with any new procedure, it is important to assess the benefits, safety, efficacy and standardization before mesotherapy could be advocated for the treatment of skin rejuvenation. [145]
Although it is an easy method to perform, side effects depend on the product used; bruising and edema are common due to the inflammatory response provoked by some of the chemicals used in mesotherapy. Following mesotherapy, atypical mycobacterial infections have been reported at sites of injections necessitating antimycobacterial therapy. [146,149–151]
Platelet-rich Plasma. PRP is an autologous preparation of platelets in concentrated plasma. PRP is being widely applied in various medical fields including dermatology for its ability to stimulate wound healing. Further, it has been used clinically in mesotherapy for skin rejuvenation. [153] Although PRP injection is considered one of the minimally invasive procedures, it usually causes skin bruises which will result in longer downtime when compared with other minimally invasive modalities.
The α-granules of concentrated platelets secrete a variety of growth factors after being activated by aggregation enhancers. These factors including VEGF, PDGF IGF and TGF are known to control cell migration, attachment, proliferation as well as cell differentiation with improving the production of ECM protein by binding to certain cell surface receptors. [154,155]
Since these growth factors are present in high concentrations, PRP has been used in different cosmetic surgeries as well as clinical treatments including challenging surgical wounds. [156] As PRP secretes a variety of growth factors in order to regenerate the skin, it may be hypothesized that PRP may promote new collagen synthesis as well as other ECM components through activation of fibroblasts; accordingly it is used to rejuvenate photoaged facial skin with consequential improving in its clinical appearance. [157,158] The secretion of various growth factors is triggered by the activation of platelets after its coagulation resulting in enhancing the mitogenic effects in different cell types. [159,160]
Up to date, there is no evidently clear method for the clinical application of PRP. Different methods are being tested including topical application or direct injection into the skin. The use of microneedling and lasers are another approach for enhancing skin remodeling by increasing penetration and producing mild inflammatory reactions. Meanwhile, the evidence-based anti-aging effects of this modality in vivo remain to be determined. Further studies are essential to conclude whether such procedures produce beneficial effects in aged skin. [153,161]
Stem Cell Therapy & Stem Cell Factors. The use of body’s stem cells and growth factors is another therapeutic modality for repair of damaged tissue, and cell-based therapy. The study of stem cells in dermatology is a rapidly emerging field in both basic and clinical research. [162]
Tissue-derived or adipose-derived stem cells (ADSCs) display multilineage developmental plasticity and secrete various growth factors, similar to fibroblasts action, such as IGF, VEGF and transforming growth factor-beta1 (TGF-β1), thus it was hypothesized that ADSCs may improve photodamaged skin. [163] Tissue-derived stem cells and its secretory factors have been shown to protect dermal fibroblasts from oxidative stress caused by ultraviolet radiation and chemicals. Studies also showed that the injection of ADSCs and adipose-derived stem cell conditioned media (ADSC-CM) stimulate migration of dermal fibroblasts with subsequent collagen synthesis during wound healing and reduce wrinkles. [44,164]
Advantage of this modality is the demonstrated safety and effectiveness of stem cells in repairing damaged tissue, because the pathophysiology of photoaging is similar to that of chronic wounds. In addition, ADSC-CM has been shown to increase protein expression of type I collagen and reduce the protein level of matrix metalloproteinases (which degrade collagen) in fibroblasts. [165,166]
Expert Commentary & Five-year View
The armamentarium offered for minimally invasive facial rejuvenation is strikingly expanding, however because each person is different, there is no one modality that is suitable for everybody. Thus, it is important not only to correct the wrinkles, but also to develop a cosmetic overall concept with the patient. This suggests that the choice of any of the treatment options should be selected according to the patient’s condition, needs and goals.
BTX injection may be useful for wrinkles associated with mild and early degrees of photodamage in young patients with hyperkinetic muscles, while lasers, light sources and RF technologies are more beneficial for more photodamage caused by loss of collagen. Meanwhile, combination therapy is highly useful for those having moderate to severe photodamaged skin with hyperkinetic muscles or volume loss. For appropriate reversal of signs of aging face, selection of a suitable combination should be put in mind. A combination treatment is considered an approach that includes at least two different and unrelated modalities, such as a light or laser device combined with non-laser technology, ELOS or other procedures or techniques. Often, appropriate patient selection and combination of different techniques allow individualized treatment with optimal outcomes.
Multiple sessions and combined minimally invasive modalities, beside the use of future home devices would fill the gap between ablative and non-ablative approaches in improving the signs of skin aging and maintaining the clinical and the histological improvement. Advanced cellular and skin aging knowledge and research paves the way for the technical evolution of the use of stem cells in skin rejuvenation. Meanwhile, advances of laser sources and techniques, fractional laser and other energy approaches, beside new and safer filler options, as well as new neurotoxin complexes represents a promising future for minimally invasive skin rejuvenation and treatment of the aging skin.
References
- Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch. Dermatol. 144(5), 666–672 (2008).
- Knaggs H. A new source of aging? J. Cosmet. Dermatol. 8(2), 77–82 (2009).
- El-Domyati M, El-Ammawi TS, Medhat W et al. Radiofrequency facial rejuvenation: evidence-based effect. J. Am. Acad.Dermatol. 64(3), 524–535 (2011).
** Evaluates the clinical effect and objectively quantifies the histologic changes of monopolar radiofrequency (RF) in the treatment of skin aging. - Kligman AM. Early destructive effect of sunlight on human skin. JAMA 210(13), 2377–2380 (1969).
- Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. J. Investig. Dermatol. Symp.Proc. 7(1), 51–58 (2002).
- Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J. Drugs Dermatol. 7(2 Suppl.), s12–16 (2008).
- El-Domyati M, Attia S, Saleh F, Ahmad H, Uitto J. Effect of topical tretinoin on photoaged facial skin: a histometric, immunohistochemical and ultrastructural study. J. Cosmet. Dermatol. 3(4), 191–201 (2004).
- Kim KH, Geronemus RG. Nonablative laser and light therapies for skin rejuvenation. Arch. Facial Plast. Surg. 6(6), 398–409 (2004).
- Mandy SH. Satisfying patient expectations with soft-tissue augmentation. Dermatol.Online J. 15(7), 1 (2009).
- El-Domyati M, El-Ammawi TS, Medhat W et al. Electro-optical synergy technique: a new and effective nonablative approach to skin aging. J. Clin. Aesthet. Dermatol. 3(12), 22–30 (2010).
** Investigates the effect of electro-optical synergy (ELOS) on connective tissue composition by histological and immunohistochemical techniques coupled with computerized morphometric analysis. - Rohrer TE. Lasers and cosmetic dermatologic surgery for aging skin. Clin.Geriatr. Med. 17(4), 769–794 (2001).
- Binder WJ, Blitzer A, Brin MF. Treatment of hyperfunctional lines of the face with botulinum toxin A. Dermatol. Surg. 24(11), 1198–1205 (1998).
- Matarasso SL. The use of injectable collagens for aesthetic rejuvenation. Semin.Cutan. Med. Surg. 25(3), 151–157 (2006).
- Bogle MA. Minimally invasive techniques for improving the appearance of the aging face. Expert Rev. Dermatol. 2(4), 427–435 (2007).
* Reviews combination of treatments used to confront the major changes associated with aging. - Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J. Am. Acad.Dermatol. 58(5), 719–737 (2008).
** Discusses the spectrum of lasers and light technologies available for skin resurfacing. - Atiyeh BS, Dibo SA. Nonsurgical nonablative treatment of aging skin: radiofrequency technologies between aggressive marketing and evidence-based efficacy. Aesth. Plast. Surg. 33(3), 283–294 (2009).
- Goldman A, Wollina U. Facial rejuvenation for middle-aged women: a combined approach with minimally invasive procedures. Clin. Interv. Aging. 5(23), 293–299 (2010).
- DeHoratius DM, Dover JS. Nonablative tissue remodeling and photorejuvenation. Clin. Dermatol. 25(5), 474–479 (2007).
- Konda D, Thappa DM. Age reversing modalities: an overview. Indian J. Dermatol.Venereol. Leprol. 79(1), 3–8 (2013).
- Roberts WE. Skin type classification systems old and new. Dermatol. Clin. 27(4), 529–533 (2009).
- Battle EJ, Soden CJ. The use of lasers in darker skin types. Semin. Cutan. Med. Surg. 28(2), 130–140 (2009).
- Hantash BM, Gladstone HB. Current role of resurfacing lasers. G. Ital. Dermatol.Venereol. 144(3), 229–241 (2009).
- Orringer JS, Voorhees JJ, Hamilton T et al. Dermal matrix remodeling after nonablative laser therapy. J. Am. Acad. Dermatol. 53(5), 405–410 (2005).
- Sadick NS. Update on non-ablative light therapy for rejuvenation: a review. LasersSurg. Med. 32(2), 120–128 (2003).
- Dierickx CC, Anderson RR. Visible light treatment of photoaging. Dermatol. Ther. 18(3), 191–208 (2005).
- Adamic M, Troilius A, Adatto M, Drosner M, Dahmane R. Vascular lasers and IPL: guidelines for care from the European Society for Laser Dermatology (ESLD). J. Cosmet. Laser Ther. 9(2), 113–124 (2007).
- Nelson JS, Majaron B, Kelly KM. What is nonablative photorejuvenation of human skin? Semin. Cutan. Med. Surg. 21(4), 238–250 (2002).
- Spendel S, Prandl EC, Schintler MV et al. Treatment of spider leg veins with the KTP (532 nm) laser–a prospective study. LasersSurg. Med. 31(3), 194–201 (2002).
- Cassuto DA, Ancona DM, Emanuelli G. Treatment of facial telangiectasias with a diode-pumped Nd:YAG laser at 532 nm. J. Cutan. Laser Ther. 2(3), 141–146 (2000).
- Weiss RA, Weiss MA, Beasley KL, Munavalli G. Our approach to non-ablative treatment of photoaging. Lasers Surg. Med. 37(1), 2–8 (2005).
- Zdinak LA, Summerfield ME. Nonablative skin therapies. Ophthalmol. Clin. North Am. 18(2), 237–248 (2005).
- Goldberg DJ, Sarradet D, Hussain M, Krishtul A, Phelps R. Clinical, histologic, and ultrastructural changes after nonablative treatment with a 595-nm flashlamp-pumped pulsed dye laser: comparison of varying settings. Dermatol. Surg. 30(7), 979–982 (2004).
- Rinaldi F. Laser: a review. Clin. Dermatol. 26(6), 590–601 (2008).
- Kishi K, Okabe K, Ninomiya R et al. Early serial Q-switched ruby laser therapy for medium-sized to giant congenital melanocytic naevi. Br. J. Dermatol. 161(2), 345–352 (2009).
- Choi JE, Kim JW, Seo SH et al. Treatment of Becker’s nevi with a long-pulse alexandrite laser. Dermatol. Surg. 35(7), 1105–1108 (2009).
- Rosenbach A, Lee SJ, Johr RH. Treatment of medium-brown solar lentigines using an alexandrite laser designed for hair reduction. Arch. Dermatol. 138(4), 547–548 (2002).
- Waibel JS. Photorejuvenation. Dermatol.Clin. 27(4), 445–457 (2009).
- El-Domyati M, El-Ammawi TS, Moawad O et al. Intense pulsed light photorejuvenation: a histological and immunohistochemical evaluation. J. DrugsDermatol. 10(11), 1246–1252 (2011).
- Weiss RA, McDaniel DH, G. GR. Review of nonablative photorejuvenation: reversal of the aging effects of the sun and environmental damage using laser and light sources. Semin. Cutan. Med. Surg. 22(2), 93–106 (2003).
- Fodor L, Carmi N, Fodor A, Ramon Y, Ullmann Y. Intense pulsed light for skin rejuvenation, hair removal, and vascular lesions: a patient satisfaction study and review of the literature. Ann. Plast. Surg. 62(4), 345–349 (2009).
- Galeckas KJ. Update on lasers and light devices for the treatment of vascular lesions. Semin. Cutan. Med. Surg. 27(4), 276–284 (2008).
- Barolet D. Light-emitting diodes (LEDs) in dermatology. Semin. Cutan. Med. Surg. 27(4), 227–238 (2008).
- Trelles MA, Mordon S, Calderhead RG. Facial rejuvenation and light: our personal experience. Lasers Med. Sci. 22(2), 93–99 (2007).
- Shamban AT. Current and new treatments of photodamaged skin. Facial Plast. Surg. 25(5), 337–346 (2009).
- Gold MH. Photodynamic therapy for cosmetic uses on the skin: an update 2010. G. Ital. Dermatol. Venereol. 145(4), 525–541 (2010).
- Akaraphanth R, Kanjanawanitchkul W, Gritiyarangsan P. Efficacy of ALA-PDT vs blue light in the treatment of acne.Photodermatol. Photoimmunol. Photomed. 23(5), 186–190 (2007).
- Karrer S, Kohl E, Feise K et al. Photodynamic therapy for skin rejuvenation: review and summary of the literature–results of a consensus conference of an expert group for aesthetic photodynamic therapy. J. Dtsch. Dermatol. Ges. 11(2), 137–148 (2013).
- Schmieder GJ, Huang EY, Jarratt M. A multicenter, randomized, vehicle-controlled phase 2 study of blue light photodynamic therapy with aminolevulinic acid HCl 20% topical solution for the treatment of actinic keratoses on the upper extremities: the effect of occlusion during the drug incubation period. J. Drugs Dermatol. 11(12), 1483–1489 (2012).
- Morton CA, Szeimies RM, Sidoroff A, Braathen LR. European guidelines for topical photodynamic therapy part 2: emerging indications – field cancerization, photorejuvenation and inflammatory/infective dermatoses. J. Eur. Acad. Dermatol.Venereol 27(6), 672–679 (2013).
- Yang G, Xiang LF, Gold MH. 5-Aminolevulinic acid-based photodynamic intense pulsed light therapy shows better effects in the treatment of skin photoaging in Asian skin: a prospective, single-blinded, controlled trial. J. Clin. Aesthet. Dermatol. 3(3), 40–43 (2011).
- Sanclemente G, Medina L, Villa JF, Barrera LM, Garcia HI. A prospective split-face double-blind randomized placebo-controlled trial to assess the efficacy of methyl aminolevulinate + red-light in patients with facial photodamage. J. Eur.Acad. Dermatol. Venereol. 25(1), 49–58 (2011).
- Pryor L, Gordon CR, Swanson EW et al. Dermaplaning, topical oxygen, and photodynamic therapy: a systematic review of the literature. Aesthetic. Plast. Surg. 35(6), 1151–1159 (2011).
- Xi Z, Shuxian Y, Zhong L et al. Topical 5-aminolevulinic acid with intense pulsed light versus intense pulsed light for photodamage in Chinese patients. Dermatol.Surg. 37(1), 31–40 (2011).
- Goldberg DJ. Nonablative dermal remodeling: does it really work? Arch.Dermatol. 138(10), 1366–1368 (2002).
- Hardaway CA, Ross EV. Nonablative laser skin remodeling. Dermatol. Clin. 20(1), 97–111 (2002).
- Dang Y, Ren Q, Liu H, Zhang J. Effects of the 1,320-nm Nd:YAG laser on transepidermal water loss, histological changes, and collagen remodeling in skin. Lasers Med. Sci. 21(3), 147–152 (2006).
- Elsaie ML, Lloyd HW. Latest laser and light-based advances for ethnic skin rejuvenation. Indian J. Dermatol. 53(2), 49–53 (2008).
- El-Domyati M, El-Ammawi TS, Medhat W et al. Effects of the Nd:YAG 1320-nm laser on skin rejuvenation: clinical and histological correlations. J. Cosmet. LaserTher. 13(3), 98–106 (2011).
- Doshi SN, Alster TS. 1,450 nm long-pulsed diode laser for nonablative skin rejuvenation. Dermatol. Surg. 31(9 Pt 2), 1223–1226 (2005).
- Nouri K, Ballard CJ. Laser therapy for acne. Clin. Dermatol. 24(1), 26–32 (2006).
- Doherty SD, Doherty CB, Markus JS, Markus RF. A Paradigm for facial skin rejuvenation. Facial Plast. Surg. 25(4), 245–251 (2009).
- Fournier N, Dahan S, Barneon G et al. Nonablative remodeling: clinical, histologic, ultrasound imaging, and profilometric evaluation of a 1540 nm Er:glass laser. Dermatol. Surg. 27(9), 799–806 (2001).
- Rivera AE. Acne scarring: a review and current treatment modalities. J. Am. Acad.Dermatol. 59(4), 659–676 (2008).
- Grema H, Greve B, Raulin C. Facial rhytides–subsurfacing or resurfacing? A review. Lasers Surg. Med. 32(5), 405–412 (2003).
- El-Domyati M, El-Ammawi TS, Medhat W et al. Multiple minimally invasive erbium: YAG laser mini-peels for skin rejuvenation: an objective assessment. J. Cosmet. Dermatol. 11(2), 122–130 (2012).
- Kaufman R, Hibst R. Pulsed 2.94 um Er: YAG laser skin ablation-experimental results and first clinical application.Clin. Exp.Dermatol. 15(4), 389–393 (1990).
- Majaron B, Kelly KM, Park HB, Verkruysse W, Nelson JS. Er:YAG laser skin resurfacing using repetitive long-pulse exposure and cryogen spray cooling: i. histological study. Lasers Surg. Med. 28(2), 121–130 (2001).
- Majaron B, Plestenjak P, Lukac M. Quantitative investigation of thermal damage in Er:YAG laser skin resurfacing.Proc. SPIE 3245(4), 366–373 (1998).
- Majaron B, Srinivas SM, Huang HL, Nelson JS. Deep coagulation of dermal collagen with repetitive Er:YAG laser irradiation. Lasers Surg. Med. 26(2), 215–222 (2000).
- Reinisch L. Scatter-limited phototherapy: a model for laser treatment of skin. LasersSurg. Med. 30(5), 381–388 (2002).
- Drnovsek-Olup B, Beltram M, Pizem J. Repetitive Er:YAG laser irradiation of human skin: a histological evaluation.LasersSurg. Med. 35(2), 146–151 (2004).
- Christian MM. Microresurfacing using the variable-pulse erbium:YAG laser: a comparison of the 0.5- and 4-ms pulse durations. Dermatol. Surg. 29(6), 605–611 (2003).
- Kunzi-Rapp K, Dierickx CC, Cambier B, Drosner M. Minimally invasive skin rejuvenation with erbium: YAG laser used in thermal mode. Lasers Surg. Med. 38(10), 899–907 (2006).
- Goldberg DJ. Lasers for facial rejuvenation. Am. J. Clin. Dermatol. 4(4), 225–234 (2003).
- Habbema L, Verhagen R, Van Hal R, Liu Y, Varghese B. Minimally invasive non-thermal laser technology using laser-induced optical breakdown for skin rejuvenation. J. Biophotonics 5(2), 194–199 (2012).
- El-Domyati M, Abd-El-Raheem T, Abdel-Wahab H et al. Fractional versus ablative erbium:yttrium-aluminum-garnet laser resurfacing for facial rejuvenation: an objective evaluation. J. Am. Acad.Dermatol. 68(1), 103–112 (2013).
- Narurkar VA. Nonablative fractional resurfacing in the male patient. Dermatol.Ther. 20(6), 430–435 (2007).
- Reddy BY, Hantash BM. Emerging technologies in aesthetic medicine. Dermatol. Clin. 27(4), 521–527 (2009).
- Fitzpatrick R, Geronemus R, Goldberg D et al. Multicenter study of noninvasive radiofrequency for periorbital tissue tightening. Lasers Surg. Med. 33(4), 232–242 (2003).
- Narurkar VA. Nonablative fractional laser resurfacing. Dermatol. Clin. 27(4), 473–483 (2009).
- Geraghty LN, Biesman B. Clinical evaluation of a single wavelength fractional laser and a novel multi-wavelength fractional laser in the treatment of photodamaged skin. Lasers Surg. Med. 41(6), 408–416 (2009).
- Laubach HJ, Tannous Z, Anderson RR, Manstein D. Skin responses to fractional photothermolysis. Lasers Surg. Med. 38(2), 142–149 (2006).
- Jih MH, Goldberg LH, Kimyai-Asadi A. Fractional photothermolysis for photoaging of hands. Dermatol. Surg. 34(1), 73–78 (2008).
- Graber EM, Tanzi EL, Alster TA. Side effects and complications of fractional photothermolysis. Experience with 961 treatments. Dermatol. Surg. 34(4), 301–307 (2008).
- Datta HS, Mitra SK, Paramesh R, Patwardhan B. Theories and management of aging: modern and ayurveda perspectives. Evid. Based Complement. Alternat. Med. 10(1), 1–6 (2011).
- Lolis MS, Goldberg DJ. Radiofrequency in cosmetic dermatology: a review. Dermatol.Surg. 38(11), 1765–1776 (2012).
* Summarizes the various types of RF devices and their uses and determines the evidence-based efficacy of these devices. - De Felipe I, Del Cueto SR, Perez E, Redondo P. Adverse reactions after nonablative radiofrequency: follow-up of 290 patients. J. Cosmet. Dermatol. 6(3), 163–166 (2007).
- Biesman BS, Pope K. Monopolar radiofrequency treatment of the eyelids: a safety evaluation. Dermatol. Surg. 33(2), 794–801 (2007).
- Alster TS, Lupton JR. Nonablative cutaneous remodeling using radiofrequency devices. Clin. Dermatol. 25(5), 487–491 (2007).
- Bogle MA, Dover JS. Tissue tightening technologies. Dermatol. Clin. 27(4), 491–499 (2009).
- Jay A. Burns thermage: monopolar radiofrequency. Aesth. Surg. J. 25(2), 638–642 (2005).
- Ruiz-Esparza J. Nonablative radiofrequency for facial and neck rejuvenation. A faster, safer, and less painful procedure based on concentrating the heat in key areas: the ThermaLift concept. J. Cosmet. Dermatol. 5(1), 68–75 (2006).
- Bassichis BA, Dayan S, Thomas JR. Use of a nonablative radiofrequency device to rejuvenate the upper one-third of the face. Otolaryngol. Head Neck Surg. 130(40), 397–406 (2004).
- Bogle MA, Ubelhoer N, Weiss RA, Mayoral F, Kaminer MS. Evaluation of the multiple pass, low fluence algorithm for radiofrequency tightening of the lower face. Lasers Surg. Med. 39(3), 210–217 (2007).
- Taylor MB, Prokopenko I. Split-face comparison of radiofrequency versus long-pulse Nd-YAG treatment of facial laxity.J. Cosmet. Laser Ther. 8(1), 17–22 (2006).
- Alhalabi SM, Agha OQ, Hantash BM. Nonablative radiofrequency for skin rejuvenation: a review of the literature.Expert Rev. Dermatol. 7(6), 589–599 (2012).
* Reviews the outcomes of the recent studies and the latest developments in non-ablative RF skin rejuvenation. - Winstanley DA, Uebelhoer NS. Future considerations in cutaneous photomedicine. Semin. Cutan. Med. Surg. 27(4), 301–308 (2008).
- Stewart N, Lim AC, Lowe PM, Goodman G. Lasers and laser-like devices: part one. Australas. J. Dermatol. 54(3), 173–183 (2013).
- Lee HS, Jang WS, Cha YJ et al. Multiple pass ultrasound tightening of skin laxity of the lower face and neck.Dermatol. Surg. 38(1), 20–27 (2011).
- Solish N, Lin X, Axford-Gatley RA, Strangman NM, Kane M. A randomized, single-blind, postmarketing study of multiple energy levels of high-intensity focused ultrasound for noninvasive body sculpting. Dermatol. Surg. 38(1), 58–67 (2012).
- Sadick NS. Overview of ultrasound-assisted liposuction, and body contouring with cellulite reduction. Semin. Cutan. Med.Surg. 28(4), 250–256 (2009).
- Sadick NS. Combination radiofrequency and light energies: electro-optical synergy technology in esthetic medicine.Dermatol.Surg. 31(9 Pt 2), 1211–1217 (2005).
- Bitter PJ, Stephen Mulholland R. Report of a new technique for enhanced non-invasive skin rejuvenation using a dual mode pulsed light and radio-frequency energy source: selective radio-thermolysis. J. Cosmet.Dermatol. 1(3), 142–143 (2002).
- Alhalabi SM, Hantash BM. Nonablative skin tightening: a review of the literature. Dermatol. Surg. 2(3), 5–21 (2009).
* Shed light on the technical aspects and clinical outcomes of the most promising non-ablative lasers. - Trelles MA, Allones I, Velez M. Non-ablative facial skin photorejuvenation with an intense pulsed light system and adjunctive epidermal care. Lasers Med. Sci. 18(2), 104–111 (2003).
- Hammes S, Greve B, Raulin C. Electro-optical synergy (ELOS) technology for non-ablative skin rejuvenation: a preliminary prospective study. J. Eur.Acad. Dermatol. Venereol. 20(9), 1070–1075 (2006).
- Kist D, Burns AJ, Sanner R, Counters J, Zelickson B. Ultrastructural evaluation of multiple pass low energy versus single pass high energy radio-frequency treatment. Lasers Surg. Med. 38(2), 150–154 (2006).
- Kim JE, Chang S, Won CH et al. Combination treatment using bipolar radiofrequency-based intense pulsed light, infrared light and diode laser enhanced clinical effectiveness and histological dermal remodeling in Asian photoaged skin. Dermatol. Surg. 38(1), 68–76 (2012).
- Sadick NS, Makino Y. Selective electro-thermolysis in aesthetic medicine: a review. Lasers Surg. Med. 34(2), 91–97 (2004).
- Ogden S, Griffiths TW. A review of minimally invasive cosmetic procedures. Br.J. Dermatol. 159(3), 1036–1050 (2008).
** Presents evidence for the use of techniques which can easily be incorporated into outpatient dermatology practice with low overhead expenditure. - Glaser DA, Patel U. Enhancing the eyes: use of minimally invasive techniques for periorbital rejuvenation. J. Drugs Dermatol. 9(8 Suppl.), s118–128 (2010).
- El-Domyati M, Attia S, Saleh F et al. Effect of topical tretinoin, chemical peeling and dermabrasion on p53 expression in facial skin. Eur. J. Dermatol. 13(5), 433–438 (2003).
- El-Domyati M, Attia S, Saleh F, Ahmad H, Uitto J. Trichloroacetic acid peeling versus dermabrasion: a histometric, immunohistochemical, and ultrastructural comparison. Dermatol. Surg. 30(2 pt 1), 179–188 (2004).
- Fischer TC, Perosino E, Poli F, Viera MS, Dreno B. Chemical peels in aesthetic dermatology: an update 2009. J. Eur. Acad.Dermatol. Venereol. 24(3), 281–292 (2009).
- Aust MC, Fernandes D, Kolokythas P, Kaplan HM, Vogt PM. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles and skin laxity. Plast. Reconstr. Surg. 121(2), 1421–1429 (2008).
- Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin. Dermatol. 26(2), 192–199 (2008).
- Alkhawam L, Alam M. Dermabrasion and microdermabrasion. Facial Plast. Surg. 25(5), 301–310 (2009).
- Karimipour DJ, Karimipour G, Orringer JS. Microdermabrasion: an evidence-based review. Plast. Reconstr. Surg. 125(1), 372–377 (2010).
- Grimes PE. Microdermabrasion. Dermatol.Surg. 31(9 Pt 2), 1160–1165 (2005).
- Bhalla M, Thami GP. Microdermabrasion: reappraisal and brief review of literature. Dermatol. Surg. 32(6), 809–814 (2006).
- Savardekar P. Microdermabrasion. Indian J.Dermatol. Venereol. Leprol. 73(4), 277–279 (2007).
- Briden E, Jacobsen E, Johnson C. Combining superficial glycolic acid (alphahydroxy acid) peels with microdermabrasion to maximize treatment results and patient satisfaction. Cutis. 79(1 Suppl.), 13–16 (2007).
- Kempiak SJ, Uebelhoer N. Superficial chemical peels and microdermabrasion for acne vulgaris. Semin. Cutan. Med. Surg. 27(3), 212–220 (2008).
- Monheit GD, Baumann LS, Gold MH et al. Novel hyaluronic acid dermal filler: dermal gel extra physical properties and clinical outcomes. Dermatol. Surg. 36(3), 1833–1841 (2010).
- Smith SR, Jones D, Thomas JA, Murphy DK, Beddingfield Fr. Duration of wrinkle correction following repeat treatment with Juvéderm hyaluronic acid fillers. Arch. Dermatol. Res. 302(10), 757–762 (2010).
- Beer K, Lupo MP. Making the right choices: attaining predictable aesthetic results with dermal fillers. J. DrugsDermatol. 9(5), 458–465 (2010).
- Wortsman X, Wortsman J, Orlandi C et al. Ultrasound detection and identification of cosmetic fillers in the skin. J. Eur. Acad.Dermatol. Venereol. 26(3), 292–301 (2011).
- Carruthers J, Cohen SR, Joseph JH, Narins RS, Rubin M. The science and art of dermal fillers for soft-tissue augmentation. J. Drugs Dermatol. 8(4), 335–350 (2009).
- Grippaudo FR, Mattei M. High-frequency sonography of temporary and permanent dermal fillers. Skin Res. Technol. 16(3), 265–269 (2010).
- Niamtu J 3rd. Complications in fillers and Botox. Oral Maxillofac. Surg. Clin. NorthAm. 21(1), 13–21 (2009).
- Patel U, Fitzgerald R. Facial shaping: beyond lines and folds with fillers. J. DrugsDermatol. 9(8), s129–137 (2010).
- Beer K. Dermal fillers and combinations of fillers for facial rejuvenation. Dermatol. Clin. 27(4), 427–432 (2009).
- Berbos ZJ, Lipham WJ. Update on botulinum toxin and dermal fillers. Curr.Opin. Ophthalmol. 21(5), 387–395 (2010).
- Lowe NJ, Shah A, Lowe PL, Patnaik R. Dosing, efficacy and safety plus the use of computerized photography for botulinum toxins type A for upper facial lines. J. Cosmet. Laser Ther. 12(2), 106–111 (2010).
- Gordon RW. Botox cosmetic for lip and perioral enhancement. Dent. Today 28(5), 94–97 (2009).
- Bowler PJ. Dermal and epidermal remodeling using botulinum toxin type A for facial, non reducible, hyperkinetic lines: two case studies. J. Cosmet. Dermatol. 7(3), 241–244 (2008).
- Carruthers A, Carruthers J. Botulinum toxin products overview. Skin Therapy Lett. 13(6), 1–4 (2008).
- Doddaballapur S. Microneedling with dermaroller. J. Clin. Aesthet. Dermatol. 2(2), 110–111 (2009).
- Clementoni MT, Broscher M, Munavalli GS. Photodynamic photorejuvenation of the face with a combination of microneedling, red light, and broadband pulsed light. Lasers Surg.Med. 42(2), 150–159 (2010).
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J. Cutan. Aesthet. Surg. 2(1), 26–30 (2009).
- Badran MM, Kuntsche J, Fahr A. Skin penetration enhancement by a microneedle device (Dermaroller) in vitro: Dependency on needle size and applied formulation. Eur.J. Pharm. Sci. 36(4–5), 511–523 (2009).
- Arora S, Bhandaree Gupta P. Automated microneedling device – a new tool in dermatologist’s kit. J. Pak. Assoc. Dermatol. 22(4), 354–357 (2012).
- Kim JH, Park HY, Jung M, Choi EH. Automicroneedle therapy system combined with topical tretinoin shows better regenerative effects compared with each individual treatment. Clin. Exp. Dermatol. 38(1), 57–65 (2013).
- Lacarrubba F, Tedeschi A, Nardone B, Micali G. Mesotherapy for skin rejuvenation: assessment of the subepidermal low-echogenic band by ultrasound evaluation with cross-sectional B-mode scanning. Dermatol. Ther. 21(Suppl. 3), S1–S5 (2008).
- El-Domyati M, El-Ammawi TS, Moawad O et al. Efficacy of mesotherapy in facial rejuvenation: a histological and immunohistochemical evaluation. Int. J.Dermatol. 51(8), 913–919 (2012).
- Atiyeh BS, Ibrahim AE, Dibo SA. Cosmetic mesotherapy: between scientific evidence, science fiction, and lucrative business. Aesth.Plast. Surg. 32(6), 842–849 (2008).
- Iorizzo M, De Padova MP, Tosti A. Biorejuvenation: theory and practice. Clin.Dermatol. 26(2), 177–181 (2008).
- Jager C, Brenner C, Habicht J, Wallich R. Bioactive reagents used in mesotherapy for skin rejuvenation in vivo induce diverse physiological processes in human skin fibroblasts in vitro: a pilot study. Exp.Dermatol. 21(1), 72–75 (2012).
- Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review.Dermatol.Surg. 32(4), 465–480 (2006).
- Toledo LS. Emerging techniques in aesthetic plastic surgery. Clin. Plast. Surg. 36(2), 177–180 (2009).
- Vedamurthy M. Mesotherapy. Indian J.Dermatol. Venereol. Leprol. 73(1), 60–62 (2007).
- Brown SA. The science of mesotherapy: chemical anarchy. Aesth. Surg. J. 26(1), 95–98 (2006).
- Kim DH, Je YJ, Kim CD et al. Can platelet-rich plasma be used for skin rejuvenation? evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann. Dermatol. 23(4), 424–431 (2011).
- Freymiller EG. Platelet-rich plasma: evidence to support its use. J. OralMaxillofac. Surg. 62(8), 1047–1048 (2004).
- Wrotniak M, Bielecki T, Gazdzik TS. Current opinion about using the platelet-rich gel in orthopaedics and trauma surgery. Ortop. Traumatol. Rehabil. 9(3), 227–238 (2007).
- Bhanot S, Alex JC. Current applications of platelet gels in facial plastic surgery. FacialPlast. Surg. 18(1), 27–33 (2002).
- Kakudo N, Minakata T, Mitsui T et al. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast. Reconstr. Surg. 122(5), 1352–1360 (2008).
- Kakudo N, Kushida S, Kusumoto K. Platelet-rich plasma: the importance of platelet separation and concentration.Plast.Reconstr. Surg. 123(3), 1135–1136 (2009).
- Kocaoemer A, Kern S, Kluter H, Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells 25(5), 1270–1278 (2007).
- Karimipour DJ, Rittie L, Hammerberg C et al. Molecular analysis of aggressive microdermabrasion in photoaged skin.Arch.Dermatol. 145(10), 1114–1122 (2009).
- Redaelli A, Romano D, Marciano A. Face and neck revitalization with platelet-rich plasma (PRP): clinical outcome in a series of 23 consecutively treated patients. J. DrugsDermatol. 9(5), 466–472 (2010).
- Charruyer A, Ghadially R. What’s new in dermatology: epidermal stem cells. G. Ital.Dermatol. Venereol. 146(1), 57–67 (2011).
- Kim WS, Park BS, Sung JH. Protective role of adipose-derived stem cells and their soluble factors in photoaging.Arch.Dermatol. Res. 301(5), 329–336 (2009).
- Kim WS, Park BS, Park SH, Kim HK, Sung JH. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J. Dermatol. Sci. 53(2), 96–102 (2009).
- Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl.Med. 1(2), 142–149 (2012).
- Lewis CJ. Stem cell application in acute burn care and reconstruction. J. WoundCare 22(1), 7–8, 10, 12–16 (2013).
Papers of special note have been highlighted as:
* of interest
** of considerable interest
Expert Rev Dermatol. 2013;8(5):565-580. © 2013 Expert Reviews Ltd.



